Research
The Cowan groups studies electro- and photocatalytic systems for the production of fuels and renewable feedstock’s. The research group is active in 4 main areas
1. Electrocatalytic and photochemical carbon dioxide reduction
(i) CO2 molecular electrocatalysts: CO2 can be electrochemically reduced to higher value products such as carbon monoxide, methanol and ethene. Small molecule electrocatalysts offer high levels of selectivity and we have been developing new catalysts (Organometallics, 2019, 386, 1224) and ways to translate these into practical devices, for example in complete electrolysers on gas diffusion electrodes in both industrial (UK patents applied) and academic (eg Electrochimica Acta, 2021, 392, 139015) projects. A significant breakthrough from the group was the development of a CO2 reduction catalyst that could operate in acids (Chem. Sci., 2016,7, 1521-1526, 2).
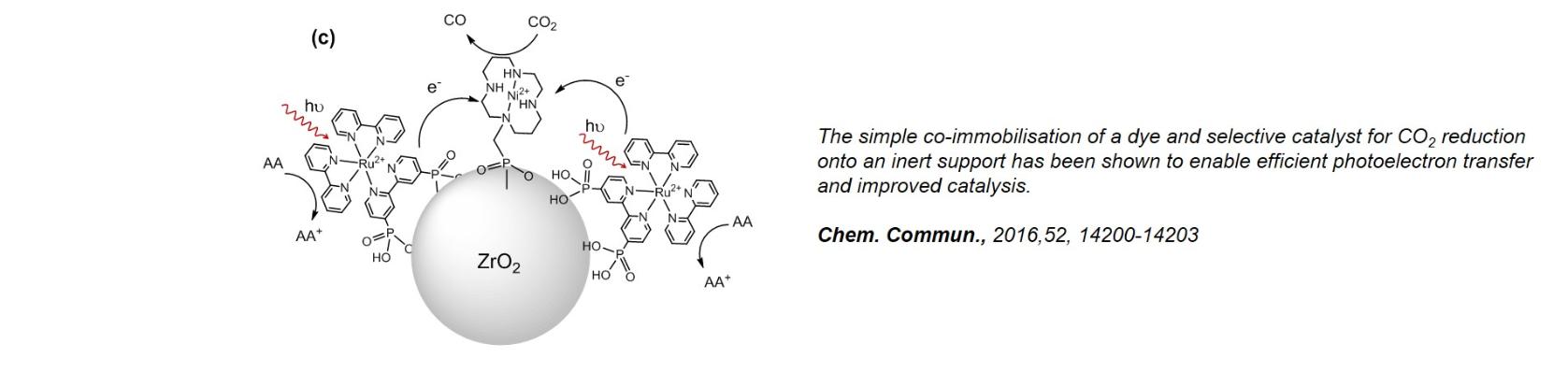
(ii) CO2 reduction photocatalysts: The direct light driven reduction of CO2 in water is challenging as proton reduction leading to hydrogen production often dominates. Our approach is to immobilise highly selective electrocatalysts onto and near to and even within the architectures visible light absorbers. Photon absorption by the sensitizer generates a high energy photoelectron which can transfer to the catalyst enabling CO2 reduction. Working with partners we have developed a wide range of systems ranging from highly active covalent organic frameworks (Chem. Sci., 2020, 11, 543), to catalyst modified quantum dots (Chem. Sci, 2018, 9, 2501) and dye-sensitized support (Chem. Commun., 2016,52, 14200)
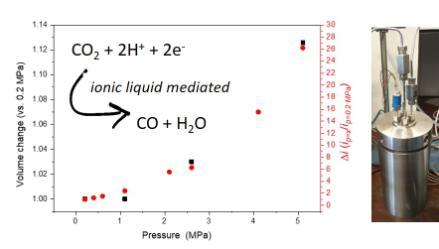
(iii) CO2 reduction in complex environments and capture media: A recent focus of the team has been to explore CO2 reduction in alternative solvents and environments, in particular posing the question - can we electrocatalytically reduce carbon dioxide within a capture medium? To date we have demonstrated how a CO2 capture microporous framework can be made electrocatalytically active (Sustainable Energy and Fuels, 2019, 3, 2990) and also demonstrated the electrocatalytic reduction of CO2 in ionic liquid/water capture media at high (>20 bar) pressures which are industrially relevant conditions (Faraday Discuss., 2021,230, 331)
2. In-situ and transient spectroscopy of electrode surfaces
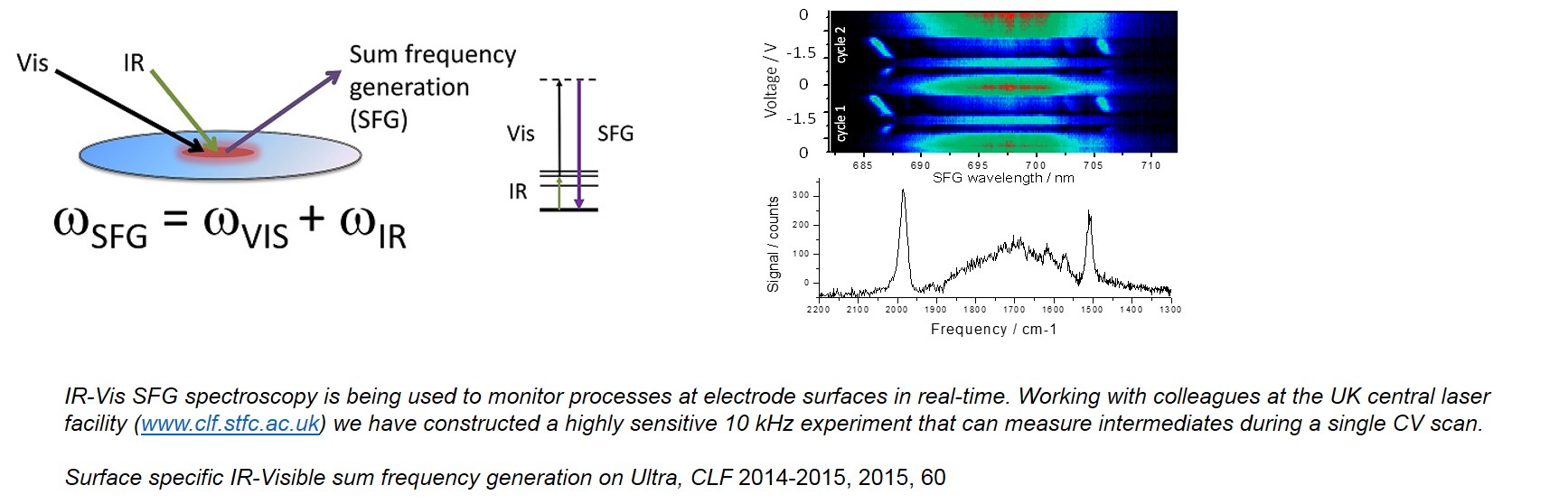
(i) Sum-Frequency Generation Spectroscopy of CO2 reduction mechanisms : SFG spectroscopy is surface/interface selective allowing us to monitor electrocatalytic mechanisms in real-time during an elcetrochemical measurement. A significant breakthrough from the group was the first studies of molecular CO2 reduction catalysts whilst they transiently interacted with the electrode surface (J. Am. Chem. Soc., 2017, 139, 39, 13791). We have since gone on to focus on group VII and VI molecular catalysts providing remarkable levels of detail and insight into catlaytic mechanisms (Nature Catalysis, 2018, 1, 952, Phys. Chem. Chem. Phys. 2019, 21, 7389). Much of the work has been in collaboration with Dr Paul Donaldson of the UK Central Laser Faccility. We are now expanding this activity to explore how this non-linear technique can also report on the local electric field at the electrode surface and to understand how this controls reactivity.
(ii) Study of water oxidation mechanisms : Using a combination of SFG and Raman spectroscopy we are exploring the mechanisms of water oxidation with benchmark electrodes and photoelectrodes. A recent highlight was our collaboration with Prof L. Hardwick at Liverpool demonstrating how the use of SHINERS nanoparticles allows for the study of IrOx catalysis mechanisms (Chem. Commun., 2020, 56, 1129)
3. Transient spectroscopy of CO2 reduction and water splitting photocatalysts/electrodes
(i) Transient absorption spectroscopy: We have 15+ yrs experience of studying charge carrier dynamics of photoelectrodes for CO2 reduction (J. Phys. Chem. C, 2017, 121 (40), 22073) and water oxidation (Angew. Chem. Int. Ed., 2016, 128, 10 3464) and recently have discussed how the community can achieve real operando measurements (J. Chem. Phys., 2020, 153, 150901). More recently, working with our collaborator (Prof Andy Cooper) we have focused on the charge carrier and excited state dynamics of organic photocatalysts with the design rules being used to help develop and understand complete water splitting systems (Angew. Chem. Int. Ed., 2020, 59, 42, 18695, J. Mater. Chem. A, 2020,8, 16283)
(ii) Time-resolved Raman spectroscopy of polymer photocatalysts: very recently the group was able to report the first use of Time-resolved resonance Raman spectroscopy on polymer photocatalysts. This is exciting as it provides a direct structural probe of the photocatalyst on the picosecond time-scale enabling determination of polaron formation pathways (J. Phys. Chem. Lett., 2021, 12, 44, 10899)
4. New photo- and electrocatalysts for water splitting
(i) Seawater electrolysis and oxygen evolution catalyst discovery: As part of the SEAFUEL program we have explored the viability of green H2 production direct from seawater (Joule, 2021, 5 ,8, 1921, Nature Energy, 2020, 5, 367). We also work with a range of collaborators on oxygen evolution catalyst discovery, in particular Dr Maulik Patel at the University of Liverpool on a range of new transition metal boride catalysts (eg ACS Appl. Energy Mater, 2020, 3, 8, 7619)
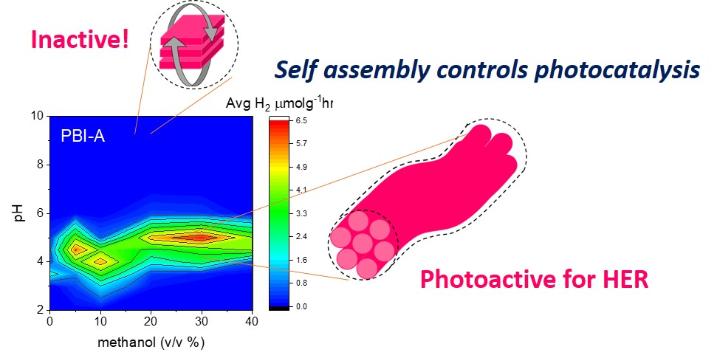
(ii) Self-assembled organic photocatalysts and photoelectrodes: Supported by the Leverhulme Trust and in collaboration with Prof Dave Adams (University of Glasgow) we are working on how self-assembly can control photophysics, and hence photocatalytic activity. Remarkable differences can be obtained by reconstructing a supramolecular structure using the same base unit chromophore! (eg Adv. Energy Mater., 2020, 2002469, Physical Chemistry Chemical Physics, 2019,21, 26466)