Publications
Storage without Strain
Aïssa, C. Storage without Strain. Nat. Synth 2023, 2, 478–480. (News & Views)
Cyclic Sulfoxonium Ylides: Synthesis and Chemospecific Reactivity in the Catalytic Alkylation of Indoles
Caiuby, C. A. D.; Vidal, L.; Burtuloso, A. C. B.; Aïssa, C. Cyclic Sulfoxonium Ylides: Synthesis and Chemospecific Reactivity in the Catalytic Alkylation of Indoles. ChemCatChem 2023, 15, e202201643.
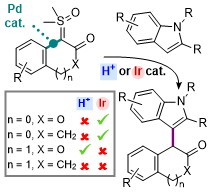
Enantioselective Intramolecular Iridium-Catalyzed Cyclopropanation of α-Carbonyl Sulfoxonium Ylides
Vidal, L.; Chen, P.-P.; Nicolas, E.; Hackett, A.; Robertson, C. M.; Houk, K. N.; Aïssa, C. Enantioselective Intramolecular Iridium-Catalyzed Cyclopropanation of α-Carbonyl Sulfoxonium Ylides. Org. Lett. 2022, 24, 8503–8508.
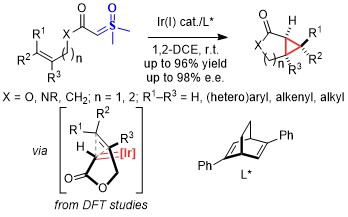
Synthesis of Sulfur-Substituted Bicyclo[1.1.1]pentanes by Iodo-Sulfenylation of [1.1.1]Propellane
Livesley, S.; Trueman, B.; Robertson, C. M.; Goundry, W. R. F.; Morris, J. A.; Aïssa, C. Synthesis of Sulfur-Substituted Bicyclo[1.1.1]pentanes by Iodo-Sulfenylation of [1.1.1]Propellane. Org. Lett. 2022, 24, 7015–7020.
-333x191.jpg)
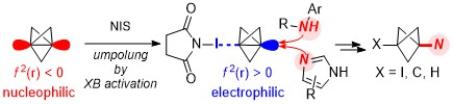
Electrophilic Activation of [1.1.1]Propellane for the Synthesis of Nitrogen-Substituted Bicyclo[1.1.1]pentanes
Livesley, S.; Sterling, A. J.; Robertson, C. M.; Goundry, W. R. F.; Morris, J. A.; Duarte, F.; Aïssa, C. Electrophilic Activation of [1.1.1]Propellane for the Synthesis of Nitrogen-Substituted Bicyclo[1.1.1]pentanes. Angew. Chem. Int. Ed. 2022, 61, e202111291. [among the most downloads in the 12 months following online publication among work published in an issue between 1 January 2021 – 31 December 2021]
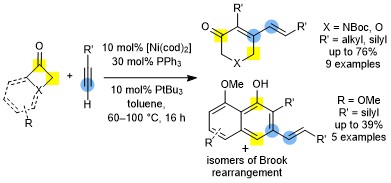
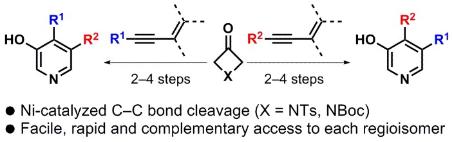
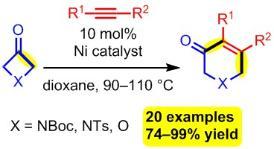
Tandem Nickel-Catalyzed Dimerization/(4+2) Cycloaddition of Terminal Alkynes with Four-Membered Ring Ketones
Barday, M.; Nicolas, E.; Higginson, B.; Delmotte, F.; Appelmans, M.; Aïssa, C. Tandem Nickel-Catalyzed Dimerization/(4+2) Cycloaddition of Terminal Alkynes with Four-Membered Ring Ketones. Synthesis 2022, 54, 1081–1090.
Palladium-Catalyzed Synthesis of α-Carbonyl-α’-(Hetero)aryl Sulfoxonium Ylides: Scope and Insight into the Mechanism
Janot, C.; Chagnoleau, J.-B.; Halcovitch, N. R.; Muir, J.; Aïssa, C. Palladium-Catalyzed Synthesis of α-Carbonyl-α’-(Hetero)aryl Sulfoxonium Ylides: Scope and Insight into the Mechanism. J. Org. Chem. 2020, 85, 1126–1137.
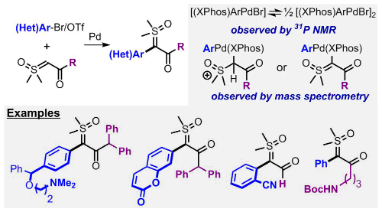
Chemospecific Cyclizations of α-Carbonyl Sulfoxonium Ylides on Aryls and Heteroaryls
Clare, D.; Dobson, B. C.; Inglesby, P. A.; Aïssa, C. Chemospecific Cyclizations of α-Carbonyl Sulfoxonium Ylides on Aryls and Heteroaryls. Angew. Chem. Int. Ed. 2019, 58, 16198–16202.
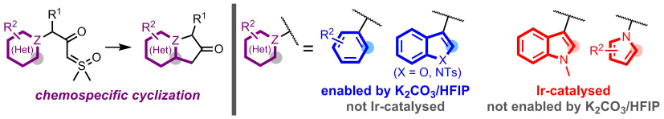
Palladium-catalyzed synthesis of bis-substituted sulfoxonium ylide
Janot, C.; Palamini, P.; Dobson, B. C.; Muir, J.; Aïssa, C. Palladium-catalyzed synthesis of bis-substituted sulfoxonium ylides. Org. Lett. 2019, 21, 296–299.
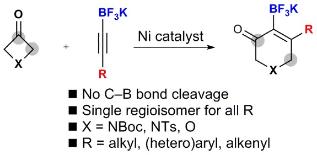
Regioselective cycloaddition of potassium alkynyltrifluoroborates with 3-azetidinones and 3-oxetanone by nickel-catalysed C–C bond activation
Elwrfalli, F.; Esvan, Y. J.; Robertson, C. M.; Aïssa, C. Regioselective cycloaddition of potassium alkynyltrifluoroborates with 3-azetidinones and 3-oxetanone by nickel-catalysed C–C bond activation. Chem. Commun. 2019, 55, 497–500.
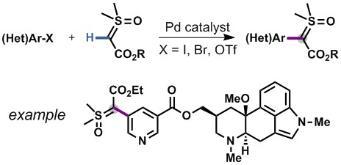
Cross-Coupling of a-Carbonyl Sulfoxonium Ylides with C–H Bonds
Barday, M.; Janot, C.; Halcovitch, N. R.; Muir, J.; Aïssa, C. Cross-Coupling of a-Carbonyl Sulfoxonium Ylides with C–H Bonds. Angew. Chem. Int. Ed. 2017, 56, 13117–13121.
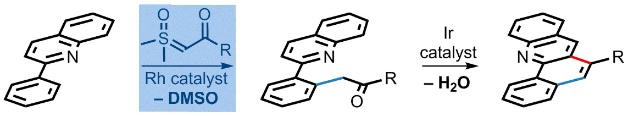
On the Regioselectivity of the Nickel-Catalyzed Insertion of Alkynes into the Carbon–Carbon Bond of Oxetan-3-one
Barday, M.; Janot, C.; Clare, D.; Carr-Knox, C.; Higginson, B.; Aïssa, C. On the Regioselectivity of the Nickel-Catalyzed Insertion of Alkynes into the Carbon–Carbon Bond of Oxetan-3-one. Synthesis 2017, 49, 3582–3589.
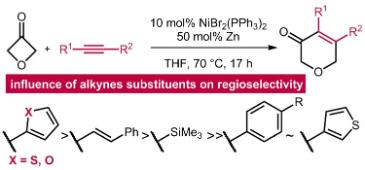
The synthesis and biological evaluation of a kabiramide C fragment modified with a WH2 consensus actin-binding motif as a potential disruptor of the actin cytoskeleton
Tetlow, D. J.; Winder, S. J.; Aïssa, C. The synthesis and biological evaluation of a kabiramide C fragment modified with a WH2 consensus actin-binding motif as a potential disruptor of the actin cytoskeleton. Chem. Commun. 2016, 52, 807–810.
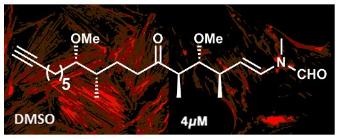
Regioselective Synthesis of 3‑Hydroxy-4,5-alkyl-Substituted Pyridines Using 1,3-Enynes as Alkynes Surrogates
Barday, M.; Ho, K. Y. T.; Halsall, C. T.; Aïssa, C. Regioselective Synthesis of 3‑Hydroxy-4,5-alkyl-Substituted Pyridines Using 1,3-Enynes as Alkynes Surrogates. Org. Lett. 2016, 18, 1756–1759.
Isomerization of Olefins Triggered by Rhodium-Catalyzed C-H Bond Activation: Control of Endocyclic beta-Hydrogen Elimination
Yip, S. Y. Y.; Aïssa, C. Isomerization of Olefins Triggered by Rhodium-Catalyzed C-H Bond Activation: Control of Endocyclic beta-Hydrogen Elimination. Angew. Chem. Int. Ed. 2015, 54, 6870–6873.
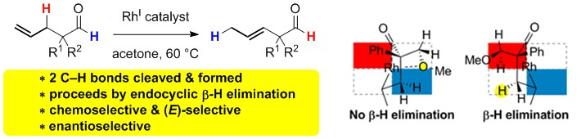
Diastereoselective Carbocyclization of 1,6-Heptadienes Triggered by Rhodium-Catalyzed Activation of an Olefinic C-H Bond
Aïssa, C.; Ho, K. Y. T.; Tetlow, D. J.; Pin-No, M. Diastereoselective Carbocyclization of 1,6-Heptadienes Triggered by Rhodium-Catalyzed Activation of an Olefinic C-H Bond. Angew. Chem. Int. Ed. 2014, 53, 4209–4212.
Transition-Metal-Catalyzed Cycloaddition of Small Ring Compounds (5.35)
Aïssa, C. Transition-Metal-Catalyzed Cycloaddition of Small Ring Compounds (5.35) in Comprehensive Organic Synthesis 2nd Edition. Eds Knochel, P.; Molander G. A.; 2014, 1738–1771. [Book chapter]
Stereoselectivity of Metal-Catalyzed Cyclizations of 1,6-Dienes
Aïssa, C. Stereoselectivity of Metal-Catalyzed Cyclizations of 1,6-Dienes. Synlett 2014, 25, 2379–2384. [Review]
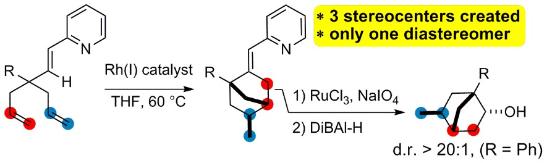
Multiple Rhodium-Catalyzed Cleavages of Single C-C Bonds
Aïssa, C.; Crépin, D.; Tetlow, D. J.; Ho, K. Y. T. Multiple Rhodium-Catalyzed Cleavages of Single C-C Bonds. Org. Lett. 2013, 15, 1322–1325.
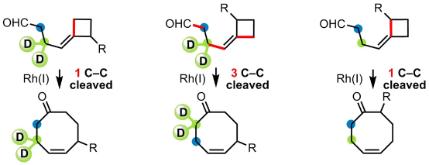
Regioselective Cycloaddition of 3-Azetidinones and 3-Oxetanones with Alkynes through Nickel-Catalysed Carbon-Carbon Bond Activation
Ho, K. Y .T.; Aïssa, C. Regioselective Cycloaddition of 3-Azetidinones and 3-Oxetanones with Alkynes through Nickel-Catalysed Carbon-Carbon Bond Activation. Chem - Eur. J. 2012, 18, 3486–3489.
Transition-Metal-Catalyzed Rearrangements of Small Cycloalkanes: Regioselectivity Trends in β-Carbon Elimination Reactions
Aïssa, C. Transition-Metal-Catalyzed Rearrangements of Small Cycloalkanes: Regioselectivity Trends in β-Carbon Elimination Reactions. Synthesis 2011, 21, 3389–3407. [Review]
Facile and chemoselective rhodium-catalysed intramolecular hydroacylation of alpha,alpha-disubtituted 4-alkylidenecyclopropanals
Crépin, D.; Tugny, C.; Murray, J.; Aïssa, C. Facile and chemoselective rhodium-catalysed intramolecular hydroacylation of alpha,alpha-disubtituted 4-alkylidenecyclopropanals. Chem. Commun. 2011, 47, 10957–10959.
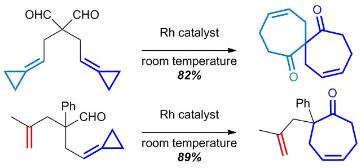
Combined Rhodium-Catalyzed Carbon-Hydrogen Activation and Beta-Carbon Elimination to access Eight-Membered Rings
Crépin, D.; Dawick, J.; Aïssa, C Combined Rhodium-Catalyzed Carbon-Hydrogen Activation and Beta-Carbon Elimination to access Eight-Membered Rings. Angew. Chem. Int. Ed. 2010, 49, 620–623.
Mechanistic Manifold and New Developments of the Julia-Kocienski Reaction
Aïssa, C. Mechanistic Manifold and New Developments of the Julia-Kocienski Reaction. Eur. J. Org. Chem. 2009, 1831–1844. [Review]
Before Liverpool
Aïssa, C.; Fürstner, A. A rhodium-catalyzed C−H activation/cycloisomerization tandem. J. Am. Chem. Soc. 2007, 129, 14836–14837
Fürstner, A.; Nevado, C.; Waser, M.; Tremblay, M.; Chevrier, C.; Teplý F.; Aïssa, C.; Moulin, E. Müller, O. Total Synthesis of Iejimalide A− D and Assessment of the Remarkable Actin-Depolymerizing Capacity of These Polyene Macrolides. J. Am. Chem. Soc. 2007, 129, 9150–9161
Fürstner A.; Kirk D.; Fenster M. D. B.; Aïssa, C.; De Souza, D.; Nevado, C.; Tuttle, T.; Thiel, W.; Müller, O. Latrunculin analogues with improved biological profiles by “diverted total synthesis. Chem. Eur. J. 2007, 13, 135–149
Fürstner, A.; Nevado, C.; Tremblay, M.; Chevrier, C.; Teplý F.; Aïssa, C.; Waser, M. Total synthesis of iejimalide B. Angew. Chem. Int. Ed. 2006, 45, 5837–5842
Fürstner, A.; Aïssa, C.; Chevrier, C.; Teplý, F.; Nevado, C.; Tremblay, M. Studies on iejimalide B: Preparation of the seco acid and identification of the molecule's “Achilles heel”. Angew. Chem. Int. Ed. 2006, 45, 5832–5837
Fürstner, A.; Aïssa, C. PtCl2-catalyzed rearrangement of methylenecyclopropanes. J. Am. Chem. Soc. 2006, 128, 6306–6307
Aïssa, C. Improved Julia− Kocienski Conditions for the Methylenation of Aldehydes and Ketones. J. Org. Chem. 2006, 71, 360–363
Fürstner A.; Kirk D.; Fenster M. D. B.; Aïssa, C.; De Souza D.; Müller, O. Diverted total synthesis: Preparation of a focused library of latrunculin analogues and evaluation of their actin-binding properties. Proc. Natl. Acad. Sci. USA 2005, 102, 8103–8108
Aïssa, C.; Riveiros, R.; Ragot, J. Fürstner A. Total syntheses of amphidinolide T1, T3, T4, and T5. J. Am. Chem. Soc. 2003, 125, 15512–15520
Fürstner, A.; Aïssa, C.; Riveiros, R.; Ragot, J. Total synthesis of amphidinolide T4. Angew. Chem. Int. Ed. 2002, 41, 4763–4766
Dhimane, A. L.; Aïssa, C.; Malacria, M. Transannular radical cascade as an approach to the diastereoselective synthesis of linear triquinane. Angew. Chem. Int. Ed. 2002, 41, 3284–3287
Aïssa, C.; Dhimane, A. L.; Malacria, M. Exploration of radical transannulations inside a cycloundecadienyne. Synlett 2000, 1585–1588
Aïssa, C.; Delouvrie, B.; Dhimane, A. L.; Fensterbank, L.; Malacria, M. New developments in radical chemistry. Applications to total synthesis and asymmetric processes. Pure&Appl. Chem. 2000, 72, 1605–1613