Tuberculosis prevention in low- and middle-income countries
Over 80% of tuberculosis infections and deaths occur in low- and middle-income countries (LMICs). Long oral regimens and poor patient acceptability contribute to ineffective and underutilised prevention therapies. Effective short courses do exist but are costly and require daily or weekly intake resulting in less than 50% completion rate despite the potential for cure.
The LONGEVITY consortium's aim for TB is to prevent the onset of TB disease by introducing an innovative prevention strategy for LMICs, which currently bear a staggering 80% of the global TB burden. This initiative aims to make a significant impact on improving healthcare outcomes.
Tuberculosis is an infectious disease caused by bacteria that often impacts a person's lungs. The World Health Organisation believe that around 25% of the planet's population have been infected by TB bacteria and that around 5-10% will develop TB disease and symptoms. Due to its connection to the lungs it is spread by coughing and sneezing of people with the infection, but people who have the bacteria but not TB disease are not infectious.
We know that TB can be fatal without treatment.
Symptoms of tuberculosis
When someone has TB disease the symptoms can be mild for a few months, so they could easily be unaware that they are spreading the disease.
Common symptoms of TB include:
- Prolonged cough (sometimes with blood)
- Weakness
- Fatigue
- Chest pain
- Fever
- Night sweats
- Weight loss.
The symptoms of TB disease can change based on where in the body TB become active. While it is often located in the lungs it can become active in other areas including the kidneys, brain, spine or skin.
The tuberculosis burden
- 80% of tuberculosis infections and deaths occur in low- and middle-income countries
- A total of 1.25 million people died from TB in 2023 (including 161,000 people with HIV)
- TB is again the biggest single infectious killer worldwide (above HIV and AIDS)
- In 2023, an estimated 10.8 million people fell ill with tuberculosis (TB) worldwide, including 6 million men, 3.6 million women and 1.3 million children
- Child and adolescent TB is often overlooked by health providers and can be difficult to diagnose and treat
- Multidrug-resistant TB remains a public health crisis and a health security threat. Only about 2 in 5 people with drug resistant TB accessed treatment in 2023
- Global efforts to combat TB have saved an estimated 79 million lives since the year 2000
- US$ 22 billion is needed annually for TB prevention, diagnosis, treatment and care to achieve the global target agreed at the 2018 UN high level-meeting on TB
- Ending the TB epidemic by 2030 is among the health targets of the United Nations Sustainable Development Goals (SDGs)
- TB is present in all countries and age groups
- TB is curable and preventable.
Source: World Health Organization: Tuberculosis
LONGEVITY and tuberculosis
Our strategy is to prevent the spread of TB in LMICs by treating latent TB infection (LTBI). In LTBI, TB bacteria are present in the body, but the immune system may be able to control their growth and prevent them from causing active disease. People with LTBI do not feel sick and cannot spread the bacteria to others. However, for some people, especially if the immune system becomes weakened (such as in people with HIV or who are receiving certain medications), the bacteria can become active and cause active TB disease. This is why people with LTBI are recommended to take TB Preventive Treatment (TPT) to get rid of the bacteria.
Currently, long oral regimens and poor patient acceptability contribute to ineffective and underutilised prevention therapies. We are targeting a one-time injectable regimen to simplify administration for patients, and healthcare programmes to reduce incidence of active disease in low- and middle-income countries (LMICs).
Effective short courses do already exist but are costly, require daily or weekly intake, and have a completion rate of less than 50%. Factors such as stigma and complexity in treatment programmes create access barriers, with patients reluctant to seek treatment.
Long-acting injectable drug delivery will contribute to reducing these barriers. Using long-acting injectable modes of drug delivery is a discreet way to take medication, thereby reducing issues related to stigma in TB affected communities.
An administration of between one and two long-acting injections may replace current oral prevention regimens which can last between one and thirty-six months, and sometimes involve taking hundreds of tablets in total. A simpler regimen will improve adherence and completion rates, thereby reducing the number of patients requiring complex therapies for active disease.
LONGEVITY's tuberculosis journey
The below information shows the foundations of the journey that LONGEVITY takes from concept to project completion for our tuberculosis work.
The map below is to show the pathway of our milestones using the green line, but the black lines show the work that also goes on simultaneously to other parts of the project. There have been and may be tweaks and changes to how this process works at different times, but the essence is there. The arrow on the map shows where we’re currently up to.
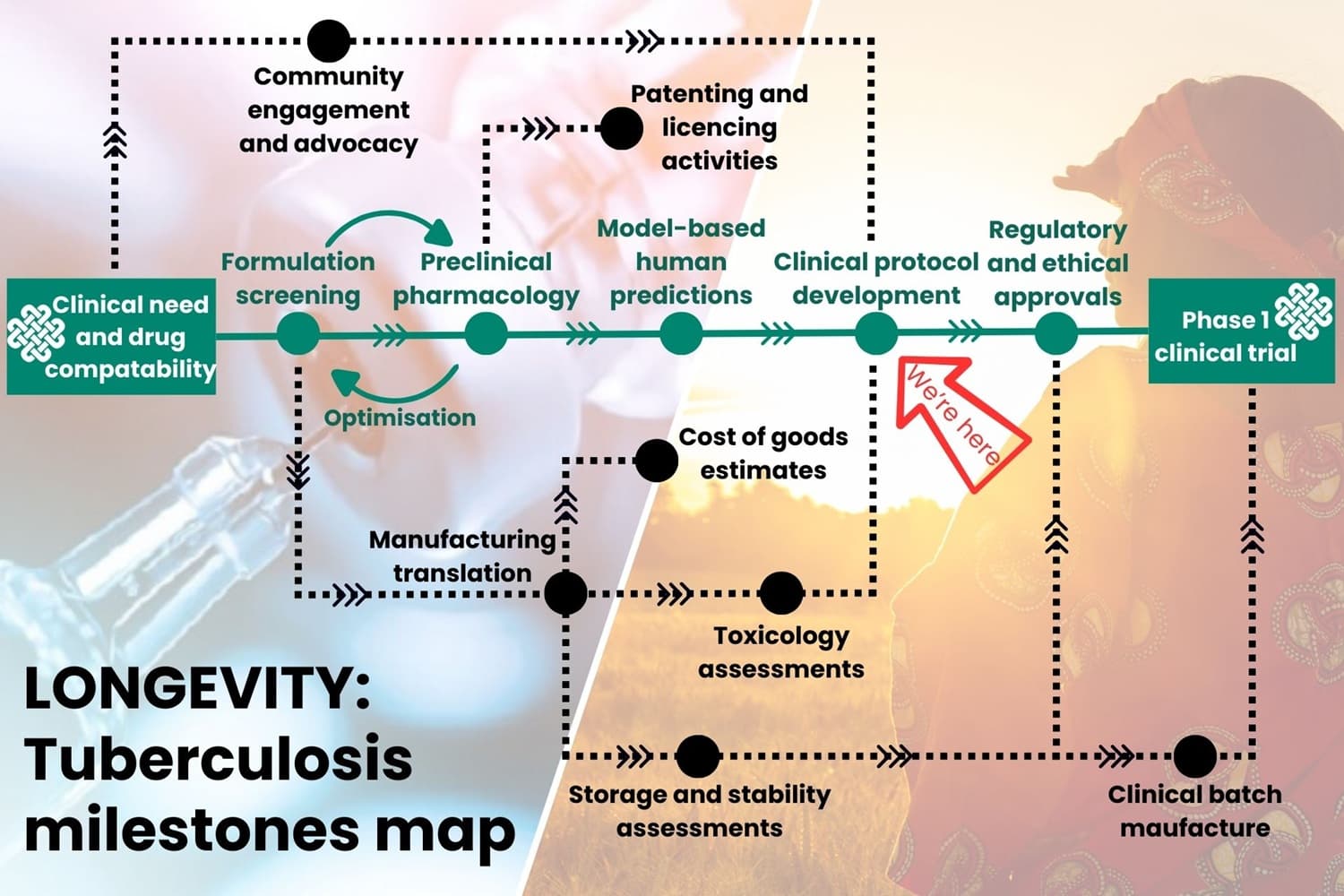
LONGEVITY tuberculosis milestones
Click below for an explanation of what each stage can entail.
Clinical need and drug compatibility
- Identify an existing, safe drug regimen that isn’t working well or could be improved through long-acting formulation to positively impact patients’ lives
- Two drugs are being taken forward - isoniazid and rifapentine.
Formulation screening
- Screen hundreds of potential formulations, following the leads that come from them
- Use currently available drugs to create formulations that are potentially long-acting
- Perform in-house stability studies on leads to make sure the formulation is stable at different temperatures over days and weeks.
Preclinical pharmacology
- Receive leads from CELT Global Health’s chemistry team
- In vitro testing and drug release testing
- identify which leads release fastest and slowest so we know the range for an in vivo study and can identify the best leads to take into animals
- In vivo study
- find the highest safest dose for a rodent, to evaluate the pharmacokinetics of the drugs
- Complete non-good laboratory practice toxicology studies to analyse PK and injection site toxicity.
- This stage is cyclical with formulation screening as the findings from one are used to optimise the other.
Model based human predictions
- Modellers use all the data alongside clinically available human oral and intravenous data, to predict a dose range that gives the best pharmacokinetics for first in human phase one trial
- Modellers work with the good laboratory practice toxicology data and compare them to the preclinical data from CELT Global Health
- They will simulate doses that would achieve the therapeutic exposure in humans
- The results from the simulation go into clinical protocol development as a recommended dose range for humans.
Clinical protocol development
- Once we know it’s stable and safe, manufacture the good manufacturing practice material for the clinical trial. This must be within the timeline for stability of the formulation as the stability clock starts ticking as soon as the first manufacture starts
- Engage with regulatory authorities prior to carrying out some aspects of the programme, such as good laboratory practice toxicology, to ensure the plan covers what is needed for progression to first-in-human trials
- Write a clinical protocol to engage with regulatory authorities to initiate first in human clinical trials (CELT Global Health and Johns Hopkins University)
- For our tuberculosis work, these will be in the United Kingdom under Medicines and Healthcare products Regulatory Agency (MHRA) regulations.
Regulatory and ethical approvals
- Clinton Health Access Initiative (CHAI)
- Work with the MHRA on regulatory paperwork required throughout clinical protocol development and manufacturing stages
- Assign a regulatory contract research organisation to submit the investigational new drug to the MHRA and manage the clinical trial.
Phase 1 clinical trial
- First doses in human volunteers.
Manufacturing translation
- Scale drug manufacture up to a kilogram format to be able to make the good manufacturing practice representative batch for good laboratory practice toxicology study
- Manufacture is completed at a contract development and manufacturing organisation.
Toxicology assessment
- The optimal formulation is administered to animals at a contract research organisation in a good laboratory practice toxicology study. This shows the safety of the long-acting injectable route and the pharmacokinetics of the drug in animals
- The information is used in clinical protocol development and informs the investigational new drug submission for the MHRA, alongside all chemistry information on the formulation.
Storage and stability assessments
- Stability trials are completed at both ambient and high temperatures, and humidity, to show how long a formulation is stable in both conditions
- This tells us how long a drug will work for before degradation starts and informs us for hot countries
- This also tells us if the drug needs to be refrigerated at the clinic, which could impact if a drug will be utilised.
Clinical batch manufacture
- A batch of formulation is manufactured within a good manufacturing practice facility at the contract development and manufacturing organisation
- The batch generated is released by a qualified person, who certifies it has been manufactured under good manufacturing practice and is safe for clinical trial.
Patenting and licencing activities
- Extentus Pharma Ltd and Medicines Patent Pool run this work from when the long-acting formulation is ready to the end of the project
- Extentus Pharma Ltd hold patents and work with Medicines Patent Pool to ensure equitable access by including prioritisation of LMICs to access licences of drug
- Extentus Pharma Ltd perform Freedom to Operate searches throughout the programme to ensure intellectual property does not infringe on others.
Cost of goods estimates
- The direct costs associated with producing a drug, including the raw materials, manufacturing, labour and packaging are estimated
- It’s a crucial metric for drug companies to assess pricing.
Community engagement and advocacy
- University of Nebraska Medical Center (UNMC) and Treatment Action Group (TAG) run this research throughout the whole project
- UNMC
- carry out surveys within communities in LMICs to see what the patients and providers think about long-acting injectables
- As the project moves along, they use more specific questions;
- preference of injecting area
- if patients would prefer to inject themselves
- TAG
- Long-acting Community Advisory Board (LAT CAB)
- TAG and members of the community such as a practitioner, a health care assistant, patients, non-infected from the community, government officials, and more, create a real representation of the community
- Discuss product, target formulation profiles
- All the findings are used to inform the application of drug development decisions.
- Long-acting Community Advisory Board (LAT CAB)
What’s Next?
LONGEVITY’s Unitaid funding ends once we reach clinical trial, however there will still be work to be done to make sure these medications reach LMIC patients in need. CELT Global Health will remain a proactive part of the work that needs to be done.
- Clinton Health Access Initiative (CHAI)
- Find a development partner (generics drug company) who will:
- Take the drug through clinical trial phase 2, which is administering doses to patient volunteers with the disease
- Take the drug through clinical trial phase 3, which is comparing the new drug to the existing drug to show that it works as well or better prioritise LMICs in need. This stage is in collaboration with LONGEVITY partner Medicines Patent Pool
- Lead commercialisation of the final product in LMICs
- File investigational new drug to MHRA and wait for approval of formulation as licensed drug.
- Find a development partner (generics drug company) who will:
Read our latest TB blogs
- Patients in low- and middle- income countries are very willing to try long-acting tuberculosis preventative injectables
- World TB Day 2025: Preparing for clinical trials
- World Tuberculosis Day 2024: An update of our work at CROI
- Stopping TB in its tracks with long-acting injectables: A war of attrition
- Reducing the burden of TB in the world’s most in need communities with long-acting injectables
- Living with TB - my experience
- Investing in TB prevention strategies with Long-Acting injectables
Subscribe to the LONGEVITY newsletter
Any personal information you provide will be held in accordance with The EU General Data Protection Regulation (GDPR). For more information please see our Privacy notice.
Our funding
The LONGEVITY Project is funded by Unitaid

The project also involves critical partners and collaborators in the Clinton Health Access Initiative,Extentus Pharma Ltd, Johns Hopkins University, Medicines Patent Pool, Treatment Action Group and the University of Nebraska Medical Center.
