SHINERS
Shine bright like a diamond
A limitation of surface enhanced Raman spectroscopy (SERS) is the restriction of being able to study only electrochemical reactions on roughened gold, copper, and silver electrodes.
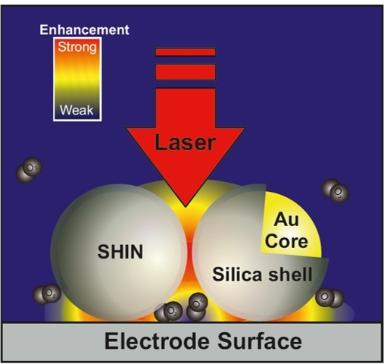
A more experimentally accessible method to achieve surface enhancement of the Raman signal at multiple types of electrode surface and morphology involves shell-isolated nanoparticles for enhanced Raman spectroscopy (SHINERS), which was first reported by Tian and co-workers from Xiamen University, China in their seminal 2010 paper reported in Nature (DOI). Here, the Raman signal amplification comes from the gold core (55 nm) which is embedded in an ultrathin (2 nm) silica shell to prevent electrocatalytic reactions at the gold nanoparticle surface.
Our research group has used SHINERS to study interfacial reactions at the electrode-electrolyte interface relating to Li-ion and metal-air batteries, water splitting and fuel cell catalysts.
As the Figure below shows you can put SHINERS onto any electrode surface in order to boost Raman sensitivity of surface reaction detection.
Group Publications, Review Articles and Book Chapters on SHINERS
Simultaneous Surface-Enhanced Raman Scattering with a Kerr Gate for Fluorescence Suppression
G. Cabello, I.V. Sazanovich, I. Siachos, M. Bilton, B.L. Mehdi, A.R. Neale, L.J. Hardwick. J.
Phys. Chem. Lett. (2024) DOI
Effect of alkali-metal cation on oxygen adsorption at Pt single-crystal electrodes in non-aqueous electrolytes
J. Fernández-Vidal, L.J. Hardwick, G. Cabello, G.A. Attard.
Faraday Discussions (2024) DOI
Long-Life and pH-Stable SnO2-Coated Au Nanoparticles for SHINERS
J. Fernández-Vidal, A. Gomez, L.A.H. Jones, C.H. Yen, T.D. Veal, V.R. Dhanak, C.-C. Hu, L.J. Hardwick.
J. Phys. Chem. C 126 (2022) 12074 DOI
Dynamics of Solid‐Electrolyte Interphase Formation on Silicon Electrodes Revealed by Combinatorial Electrochemical Screening
D. Martín-Yerga, D.Costa. Milan, X. Xu, J. Fernández-Vidal, L. Whalley, A.J. Cowan, L.J. Hardwick, P. Unwin.
Angew. Chemie. Int. Ed. (2022) e202207184 DOI
Investigating the presence of adsorbed species on Pt steps at low potentials
R. Rizo, J. Fernández-Vidal, L.J. Hardwick, G.A. Attard, F.J. Vidal-Iglesias, V. Climent, E. Herrero, J.M. Feliu.
Nature Commun. 13 (2022) 255 DOI
Water oxidation intermediates on iridium oxide electrodes probed by in situ electrochemical SHINERS
K.H. Saeed, M. Forster, J.F. Li, L.J. Hardwick, A.J. Cowan
Chemical Communications 56 (2020) 1129 DOI
Advanced spectroelectrochemical techniques to study electrode interfaces within lithium-ion and lithium-oxygen batteries
A. J. Cowan and L. J. Hardwick
Annu. Rev. Anal. Chem., 12 (2019), 23.1- 23.24 DOI
Oxygen reactions on Pt{hkl} in a non-aqueous Na+ electrolyte: Site selective stabilization of a sodium peroxy species
T. A. Galloway, J. Dong, J.F. Li, G. Attard and L. J. Hardwick
Chem. Sci, 10 (2019), 2956-2964 DOI
In situ electrochemical SHINERS investigation of SEI composition on carbon-coated Zn0.9Fe0.1O anode for lithium-ion batteries
L. Cabo-Fernandez, D. Bresser, F. Braga, S. Passerini, and L. J. Hardwick
Batteries & Supercaps., 2 (2019), 168-177 DOI
Metal-Air Batteries: Fundamentals and Applications; Chapter 9 Metal-Air Battery: In Situ Spectro-electrochemical Techniques
I. M. Aldous, L. J. Hardwick, R. J. Nichols, and J. Padmanabhan Vivek
Ed. Xin-bo Zhang, Wiley 2018, Book
Shell Isolated Nanoparticles for Enhanced Raman Spectroscopy Studies in Lithium-Oxygen Cells
T.A. Galloway, L. Cabo-Fernandez, I.M. Aldous, F. Braga, L.J. Hardwick
Faraday Discuss., 205 (2017) 469-490 DOI
Utilizing In Situ Electrochemical SHINERS for Oxygen Reduction Reaction Studies in Aprotic Electrolytes
T. Galloway, L.J. Hardwick
J. Phys. Chem. Lett., 7 (2016) 2119-2124 DOI