A degrading synthesis of proteome turnover research!
Claydon, A.C. & Beynon, R.J. (2012) Proteome dynamics: revisiting turnover with a global perspective. Mol. Cell Proteomics 11:1551-1565. [PUBMED][PDF]
Although bulk protein turnover has been measured by stable isotope labelled tracers for over half a century, it is only recently that the same approach has become applicable to the level of the proteome, permitting the analysis of turnover of many proteins instead of single proteins or an aggregated protein pool. The optimal experimental design for turnover studies is dependent on the nature of the biological system under study, which dictates the choice of precursor label, protein pool sampling strategy and treatment of data. In this review we discuss different approaches and in particular, explore how complexity in experimental design and data processing increase as we shift from unicellular to multicellular systems, in particular animals.
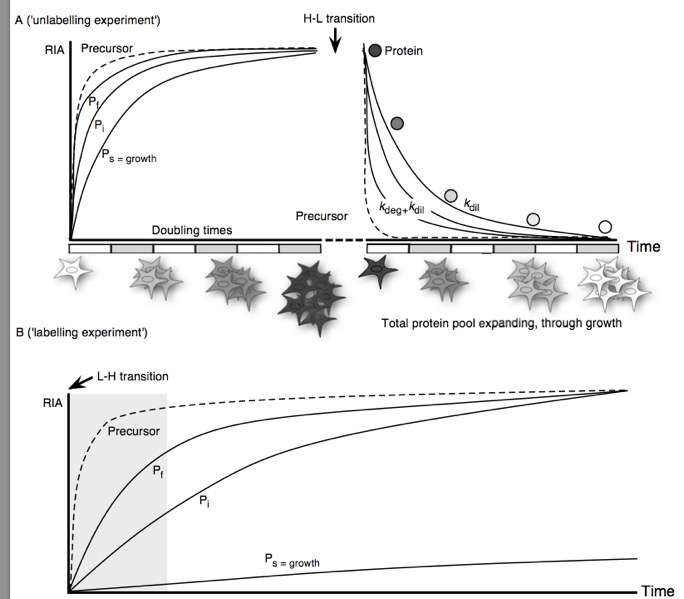
Although bulk protein turnover has been measured by stable isotope labelled tracers for over half a century, it is only recently that the same approach has become applicable to the level of the proteome, permitting the analysis of turnover of many proteins instead of single proteins or an aggregated protein pool. The optimal experimental design for turnover studies is dependent on the nature of the biological system under study, which dictates the choice of precursor label, protein pool sampling strategy and treatment of data. In this review we discuss different approaches and in particular, explore how complexity in experimental design and data processing increase as we shift from unicellular to multicellular systems, in particular animals.