Schistosome parasite QconCAT
Castro-Borgesa, W., Simpson, D.M., Dowlea, A., Curwen, R.S., Thomas-Oates, J., Beynon, R.J. & Wilson, A. R. (2011) Abundance of tegument surface proteins in the human blood fluke Schistosoma mansoni determined by QconCAT proteomics. J Proteomics (in press)
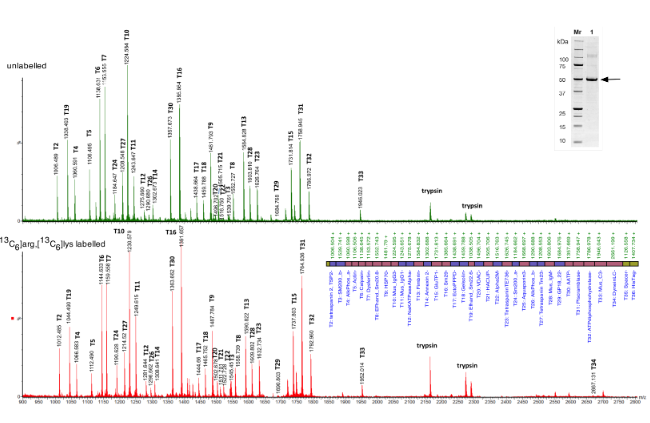
The schistosome tegument provides a major interface with the host blood stream in which it resides. Our recent proteomic studies have identified a range of proteins present in the complex tegument structure, and two models of protective immunity have implicated surface proteins as mediating antigens. We have used the QconCAT technique to evaluate the relative and absolute amounts of tegument proteins identified earlier. A concatamer comprising R- or K-terminated peptides was generated with [13C6] lysine/arginine amino acids. Two tegument surface preparations were each spiked with the purified SmQconCAT as a standard, trypsin digested, and subjected to MALDI ToF MS. The amounts of protein in the biological samples were determined by comparing the areas under the pairs of peaks, separated by 6Da, representing the light and heavy peptides derived from the biological sample and SmQconCAT, respectively. We report that aquaporin is the most abundant transmembrane protein, followed by two phosphohydrolases. Tetraspanin Tsp-2 and Annexin-2 are also abundant but transporters are scarce. Sm200 surface protein comprised the bulk of the GPI-anchored fraction and likely resides in the secreted membranocalyx. Two host IgGs were identified but in amounts much lower than their targets. The findings are related to the models of protective immunity.