Acetone modifies peptides! Be careful if you're using acetone precipitation.
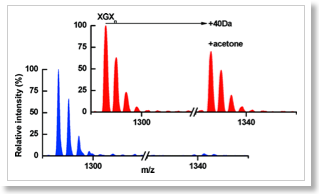
Acetone precipitation is a common method for precipitation and concentration of proteins. We show here that a trace amount of residual acetone in the precipitated protein, can, after proteolysis, lead to selective modification of peptides predominantly those in which a glycine residue is the second amino acid, probably generating a relatively stable derivative that, under gas phase conditions, generates a y(1) ion of the same mass as proline. This modification is detectable by either MALDI-ToF or ESI-ion trap mass spectrometry and under normal sample preparation conditions is incomplete. The derivatization occurs in the condensed phase and is sufficiently stable that the modified peptide can elute on reversed phase chromatography at a different time to the unmodified peptide. Acetone precipitation is such a commonly used procedure in protein sample preparation for proteomics that some caution may be warranted. A significant number of peptides (about 5% of a typical proteome) meet the requirements for this reaction and could, therefore, change the outcome of studies.