QUASAR helps pave the way for portable low cost, low dose 3D imaging
The state of the art in 3D X-ray imaging involves the use of an X-ray tube which is in some way translated and rotated in space relative to a patient. By doing so, X-ray images from 10s or 1000s of different projection angles are generated and then reconstructed in 3D. However, this mechanical movement puts a heavy toll on the time it takes to complete a 3D scan, limiting patient throughput and adding motion artefacts from the moving organs, especially the heart and the lungs. It also means that the patient is subject to a certain level of radiation.
In an open access paper just published in the journal Biomedical Physics & Engineering Express ASHE researchers from the University of Liverpool and industry partner Adaptix showed that a compact, rectangular array with several miniature cold-cathode field emitters can be used stationary, with each emitter fired electronically to generate X-ray images from different projection angles without having to slowly move a large X-ray tube.

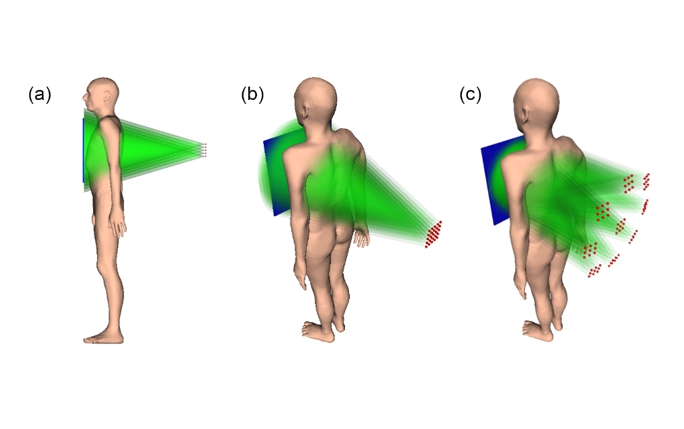
3D models of the irradiation geometry of tomosynthesis with a single flat panel source array (a, b) and of a concept irradiation geometry with a multi-panel system (c). Emitters are activated sequentially but here they are all drawn simultaneously active for illustration purposes. (Image credit: Thomas G Primidis et al 2022 Biomed. Phys. Eng. Express 8 015006 CC BY 4.0)
The technology itself is simple. Micrometre wide metallic needles inside an electric field have extremely high electric field gradients near their tip. This causes electrons on that tip to escape relatively easily (field emission) without extra power from a heated filament, as is the case with conventional X-ray tubes. Without that extra heating and with their very small size, these field emitters can be grouped in large numbers in an array the size of a tablet and with some smart electromagnetic engineering, they can be activated sequentially to send electrons towards an X-ray target. The X-ray beams that are produced originate from different positions on the target and so different projections angles needed for 3D X-ray imaging are produced without any source movements. Moreover, field emission technology requires less power to use and is much more compact, lighter and cheaper to build than conventional X-ray tubes, something that is expected to help more and smaller clinics to adopt the technology and perhaps pave the way for truly portable 3D X-ray imaging in the future.
Adaptix has used square emitter arrays at 60 kVp to get digital tomosynthesis (DT) images of small animals, teeth, human cadavers and electronic devices. DT is a modality similar to computed tomography but uses fewer projections and in a narrower angular range, offering 3D information at a lower dose and lower cost.
QUASAR Group member Thomas G Primidis and co-workers have now conceptualised an upgrade of this system from 60 kVp to 90 or 120 kVp, more suited for chest tomosynthesis. In their study, they compared the photon yield and resulting X-ray spectrum using different combinations of accelerating voltage and X-ray target thicknesses. They calculated the effective dose from doing a DT scan with emitter arrays at 90 and 120 kVp with results shown in the following figure. A significant aspect of their design was the reduced distance between source and detector to 80 cm to achieve a higher photon density.
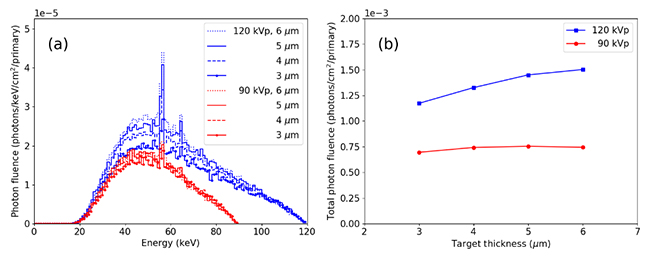
a) FLUKA generated energy spectra with source designs having different accelerating voltage and different transmissive target thickness, b) total photon fluence calculated as the area under the curve of each spectrum. (Image credit: Thomas G Primidis et al 2022 Biomed. Phys. Eng. Express 8 015006 CC BY 4.0)
Through their detailed simulations, the authors found that target thickness had only a minor effect on the width of the X-ray spectrum and that the photon yield at 120 kVp was 1.7 to 2 times that at 90 kVp. Furthermore, they demonstrated that both emitter arrays can produce 3D images of the chest and that the quality of these images was essentially identical, allowing to operate the system at lower voltages.
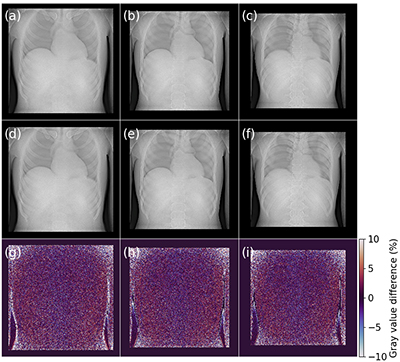
a-c) Tomosynthesis slices at different depths using the 120 kVp source array, d-f) same slices with the 90 kVp source array, g-i) relative percentage difference between 120 kVp and 90 kVp. (Image credit: Thomas G Primidis et al 2022 Biomed. Phys. Eng. Express 8 015006 CC BY 4.0)
The authors concluded that stationary, cold-cathode, flat panel X-ray source arrays are a versatile tool to create high quality 3D images of the human chest and that there is clinical benefit in reducing the accelerating voltage to only 90 kVp. Their work paves the way for more compact, low dose, low cost and possibly truly portable 3D imaging systems which are expected to benefit patients around the world.
Thomas Primidis, the lead author of the paper, said: "I am amazed by how much smaller this technology is than conventional X-ray tubes. I have seen that it works as an imaging solution for teeth and small animals, as well as a non-destructive testing tool for electronic devices. We have now demonstrated that this is also suitable for 3D chest imaging. In a next step, we will investigate multi-panel designs and how we can optimize the overall image quality.”
This work was undertaken on Barkla, part of the High-Performance Computing facilities at the University of Liverpool, UK and it was funded by the Accelerators for Security, Healthcare and Environment CDT supported by the Science and Technology Facilities Council under grant number ST/R002142/1.
Further information:
'3D chest tomosynthesis using a stationary flat panel source array and a stationary detector: A Monte Carlo proof of concept.'
Thomas G Primidis et al 2022 Biomed. Phys. Eng. Express 8 015006