From sustainable hydrocarbons to spin liquids
Published on

Fuel such as petrol is made up of hydrocarbons - a family of molecules consisting entirely of carbon and hydrogen. Pigment and dye, coal and tar are made up of hydrocarbons too.
These common, abundant materials, sometimes even associated with waste, are not often thought of as being electronically or magnetically interesting.
But an international research team led by Professor Matthew J. Rosseinsky in the University’s Department of Chemistry and Professor Kosmas Prassides of Tohoku University in Japan has made a significant find.
The team have discovered how to take such hydrocarbon molecular components, dress them with electrons, each of which carries a small compass – an unpaired spin – and pack them together like cookies in a box to create a quantum spin liquid – a long-sought hypothetical state of matter.
The existence of quantum spin liquids was first theoretically proposed in 1973. In conventional magnets, the motion of the electron spins – the tiny magnets – freezes on cooling as they align parallel or antiparallel to each other. In contrast, the spins in a quantum spin liquid never stop fluctuating, randomly and strongly, even at the lowest temperature of absolute zero.
Each individual spin points simultaneously along an infinite number of directions and is highly entangled with other spins, even those far away. As such, this sea of electron spins is predicted to be host to many exotic phenomena of both fundamental and technological interest.
However, experimental realization of this unique fully-entangled state of matter has remained to date unfulfilled. Despite a four-decade-long search, there are very few quantum spin liquid candidates. Current options include certain copper inorganic minerals and some organic salts, which contain rare, heavy or toxic elements.
In results published in two consecutive papers in the journal `Nature Chemistry’, the team came up with the new chemistry needed to make high-purity crystalline materials from the reaction of polyaromatic hydrocarbons with alkali metals for the first time.
Materials obtained from polyaromatic hydrocarbons (molecules with many aromatic rings) were proposed in the past as candidates of new superconductors – materials with no electrical resistance and able to carry electricity without losing energy – devoid of toxic or rare elements. However, destruction of the molecular components in the synthetic treatments employed had inhibited any progress in this field.
Professor Matthew Rosseinsky said: “It took us many years of work to achieve our breakthrough. But in the end, we succeeded in developing not one, but two complementary chemistry routes, which open the way to a rich variety of new materials with as-yet unknown properties.”
Professor Kosmas Prassides said: “Removing the existing synthetic roadblock has led to very exciting developments. We have already discovered that some of the structures of the new materials – made entirely of carbon and hydrogen, the simplest possible combination – show unprecedented magnetic properties – spin liquid behaviour – with potential applications in superconductivity and quantum computing.”
The Liverpool and Tohoku groups worked with teams led by Dr Ryotaro Arita at RIKEN, Japan and Professor Denis Arcon at the University of Ljubljana, Slovenia.
The research was supported by the Mitsubishi Foundation, JSPS KAKENHI, JST-ERATO Isobe Degenerate p-Integration Project, the Engineering and Physical Sciences Research Council and the European Union.
Part of the research was carried out at the synchrotron X-ray facilities at the ESRF (France) and Diamond Light Source.
The papers `π-electron S = ½ quantum-spin-liquid state in an ionic polyaromatic hydrocarbon’ (DOI: 10.1038/NCHEM.2764) and `Redox-controlled potassium intercalation into two polyaromatic hydrocarbon solids` (DOI: 10.1038/NCHEM.2765) are both published in Nature Chemistry.
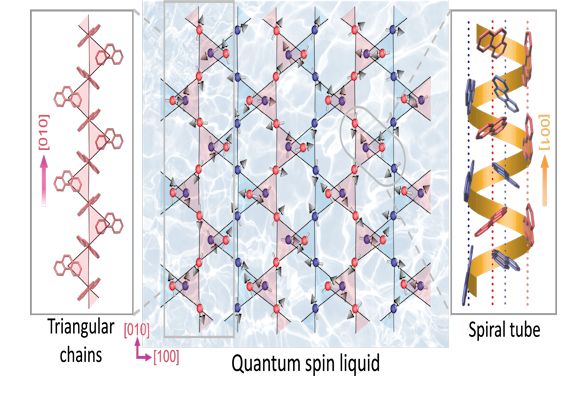
Image: Diagrammatic representation of the structure of the ionic hydrocarbon discovered in this work as host of a quantum spin liquid. The left panel shows the molecular ions, which arrange in triangular vertex-sharing chains. The right panel depicts the co-existing spiral magnetic tubes. The two structural motifs interlink to give a complex packing architecture, as shown in projection in the middle panel. Each molecular ion has one spin (shown as grey arrow). The spins perpetually fluctuate down to low temperatures. The figure shows one of an infinite number of entangled spin arrangements. © 2017 Kosmas Prassides