The outcome of refractive surgery is also affected by the same variations in corneal parameters in addition to the surgical parameters such as thickness of tissue ablation and the way the tissue integrity is influenced by the surgical procedures. Our methods attempt to consider these influences in producing more accurate IOP measurement techniques and better optimised planning of refractive surgeries.
Group studies
Our methods to improve medical procedures have resulted in algorithm that are simple to use in medical practice. Some of the methods, which have been validated experimentally on ex-vivo eyes and also clinically, have been published.
A. Planning of refractive surgery
Refractive surgery procedures are commonly used to correct the eye’s refractive errors (mainly myopia, hyperopia and astigmatism) and to eliminate or reduce the need for prescription glasses and contact lenses. Planning the surgeries currently relies on a topography subtraction procedure, which does not consider the biomechanical changes in the cornea associated with the surgeries. For example, in LASIK (the most commonly used refractive procedure), an anterior flap is cut and its connection to the residual tissue never gains its natural strength, and stromal thickness is reduced due to tissue ablation. These changes naturally reduce the mechanical stiffness of the cornea, change its response to the intraocular pressure and therefore result in a corneal shape that is different from that intended by the procedure. Our work in this area is intended to improve the planning of refractive surgery procedures by considering the biomechanical effects of the procedures. The work concentrates on LASIK and SMILE.
A.i. Predictive modelling of LASIK
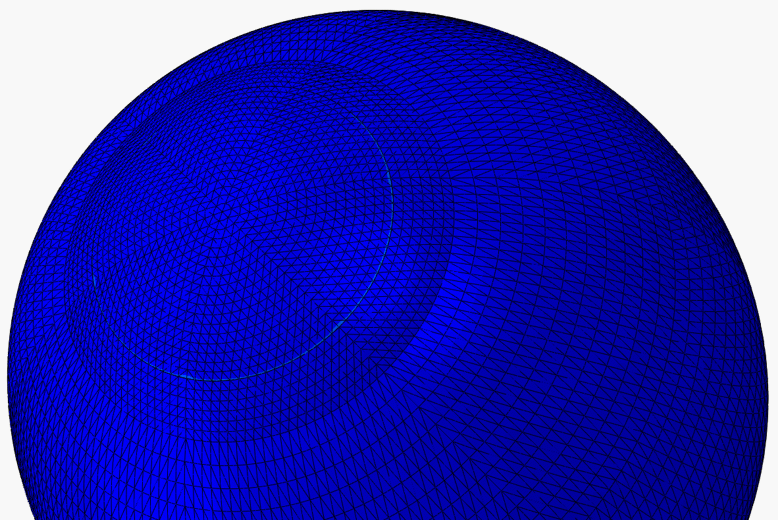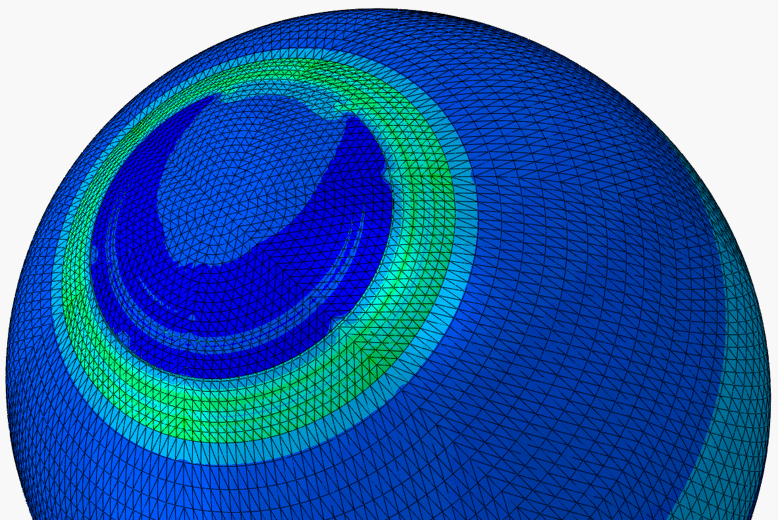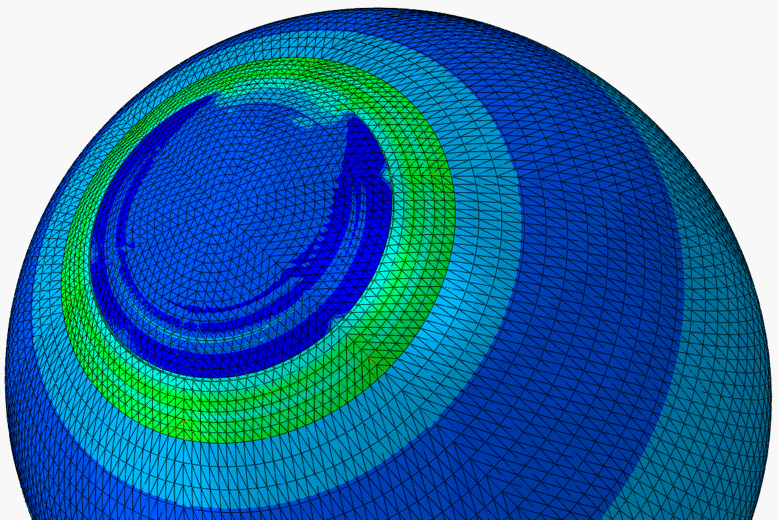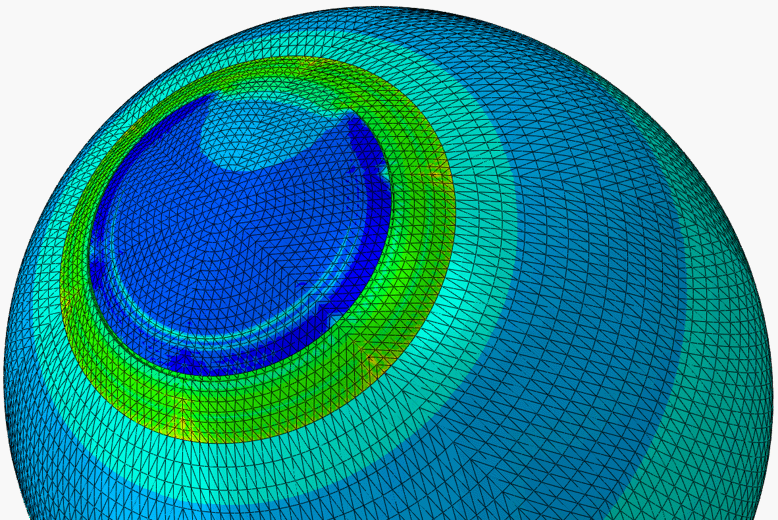
The LASIK (laser-assisted in situ keratomileusis) eye surgery is currently the most common and successful form of refractive surgery. In spite of the growing worldwide popularity of LASIK, the procedure is still in need of optimisation. As the remaining cornea post-surgery has a reduced thickness, its deformation under intraocular pressure (IOP) will change leading to a new modified topography. Lack of understanding of corneal biomechanics has meant that the analysis of the procedure and the determination of the thickness of tissue to be removed are based on average corneal properties. Significant variations in corneal thickness or material behaviour would therefore lead to failure to fully correct refractive errors and may require re-treatment. Based on experimental studies of the human eye and patient data of LASIK surgery, numerical models of the LASIK surgery are built to better understand the biomechanical behaviour of the cornea following surgery. The models can also be used to both (1) predict the outcomes of a LASIK surgery with known parameters, and (2) guide the planning of a surgical procedure based on known refractive errors.
A.ii. Predictive modelling of SMILE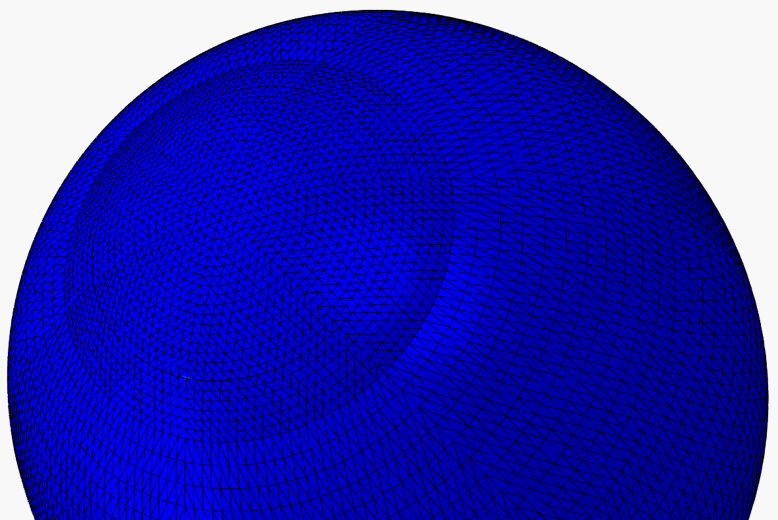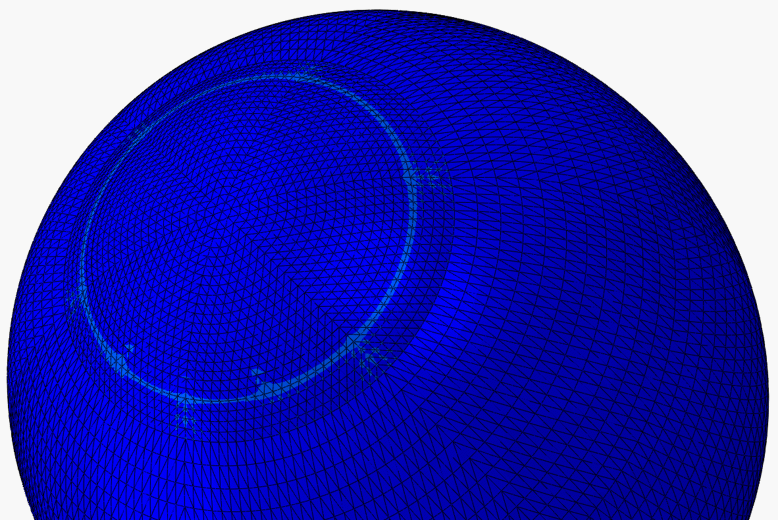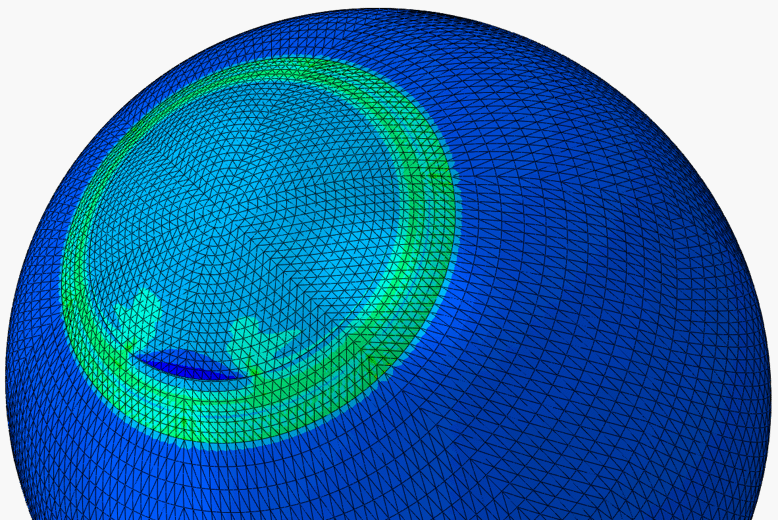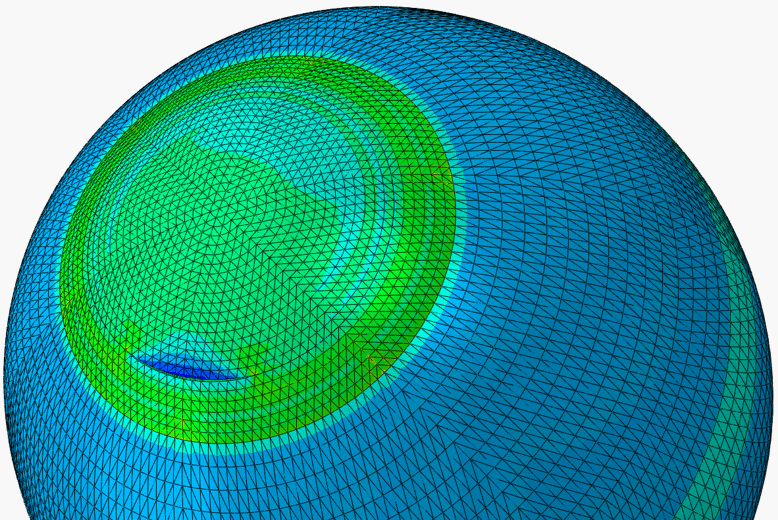
The weakening of the cornea due to the separation of the flap in LASIK has led to the development of a new form of refractive surgery called the small-incision lenticule extraction, or SMILE. In Smile, a femtosecond laser cuts a refractive lenticule within the corneal stroma. This lenticule is sucked out through a pathway created by a small incision in the anterior cornea. Since the corneal tissue on top of the lenticule remains connected to the rest of corneal tissue (apart from the area around the small incision), it remains able to contribute to the mechanical action of the cornea and hence reduces the mechanical deterioration in the cornea observed after LASIK.
In our work, numerical studies of SMILE, and subsequent validation using clinical data, have allowed comparative assessment of SMILE and LASIK and quantification of the advantages of SMILE.
B. Stiffness-corrected IOP measurements based on Corvis ST output
A numerical study has been conducted to quantify and eliminate the effect of changes in corneal mechanical stiffness on the IOP measurements made by the Corvis. The study was based on numerical simulation of the Corvis procedure, applied on human eye models with different tomographies (including thickness profiles), ages and true IOPs. The eye models were developed for analysis using the finite element method and designed to simulate important biomechanical features of the eye, including the cornea’s aspheric topography, eye’s variable thickness, epithelium’s and endothelium’s low stiffness, cornea’s weak inter-laminar adhesion and the tissue’s hyperelasticity, hysteresis, viscoelasticity and age-related stiffening. The simulations adopted a gradual application of the Corvis air pressure with the spatial distribution observed in representative laboratory experiments. The resulting deformation is producing similar output to that of the Corvis.
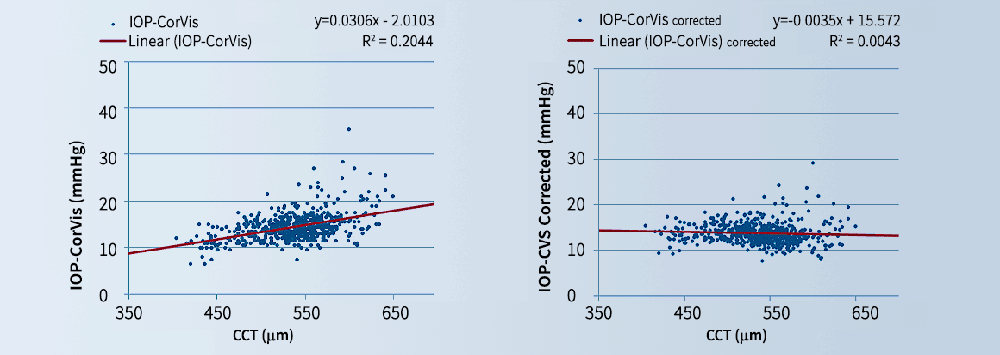
The results of the parametric numerical study were analysed to produce estimates of true IOP (IOPt) as a function of measured IOP (CVS-IOP), CCT and age. The IOPt estimates have been found in several clinical datasets to be much less correlated with corneal thickness and curvature and the patient’s age.
C. Correction of GAT IOP measurements for the effects of corneal stiffness
The Goldmann Applanation Tonometer (GAT) is the reference standard in measuring intraocular pressure (IOP). There is a large body of literature confirming the effect of variations in corneal stiffness on GAT IOP measurements (GAT-IOP). For instance, an increase in corneal stiffness or age has been found to lead to increases in GAT-IOP, resulting in false-positives or false-negatives in diagnosis and management of glaucoma. The Group has carried out a study to develop a correction algorithm of GAT-IOP that simultaneously considers corneal thickness, curvature and age. The algorithm has been assessed first experimentally (on 19 ex-vivo eyes) and second clinically (on European, Indian, African American and Chinese populations) and found in each case to reduce the association of GAT-IOP with corneal stiffness parameters.
D. Assessment of ORA as an instrument to measure IOP and predict corneal properties
The Ocular Response Analyzer (ORA) has been developed as an instrument to provide accurate estimates of IOP (that are not affected by corneal stiffness) and corneal mechanical properties. The study considered non-contact tonometers in general, i.e. those that reply on air puff to deform the cornea, and was therefore generic in nature. It used numerical simulations of both the eye and the ORA procedure and considered cases with a wide range of cornel thickness, curvature, age and values of true IOP. The findings were then validated using two clinical datasets obtained by groups at Moorfields Eye Hospital and the University of New South Wales. The study demonstrated that non-contact tonometers have the ability to accurately predict both IOP and corneal properties and identified the practical restrictions that should be considered, particularly with estimating the mechanical properties of corneal tissue.
E. Biomechanically corrected IOP measurement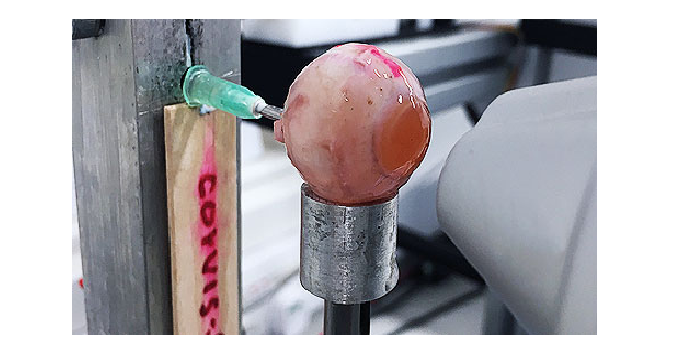
Through simulations of the Corvis procedure, and consideration of the Corvis deformation output, the new method enables the accurate estimation of an IOP that is less dependent on ocular properties, and thus biomechanically corrected IOP (bIOP). bIOP has been first tested experimentally on several ex-vivo human eye globes. The eyes were subjected to known levels of IOP and to the Corvis procedure. Both the uncorrected and corrected IOP measurements were compared with the applied IOP. In all cases, bIOP was significantly closer to true IOP than the Corvis measurement (the mean of the absolute differences between bIOP and true IOP was 0.84±0.97 mmHg compared with 3.46±1.09 mmHg for the absolute differences between uncorrected Corvis IOP and true IOP).
bIOP was then assessed clinically on a number of clinical databases involving thousands of Corvis measurements. In all cases, bIOP significantly reduced the association between IOP measurements and both corneal thickness and age. bIOP was also assessed for patients undergoing the LASIK refractive surgeries (Figure 10). The difference in bIOP taken before and after surgery was 1.0 mmHg (14.6±2.0 pre-surgery vs 13.6±2.1 post-surgery), compared with 3.4 mmHg for uncorrected Corvis readings (14.8±2.4 pre-surgery vs 11.4±1.9 post-surgery) and 3.1 mmHg for Goldmann Applanation Tonometry (GAT) readings (15.8±2.4 pre-surgery vs 12.7±2.3 post-surgery). Find our more at: www.corneal-biomechanics.de
Back to: Biomechanical Engineering Group