Regulation of Nutritional Sensing by PI3K Family Enzymes and GCN2
- Bahram Ebrahimi
- Suitable for: Staff and students with an interest in Genomes, Systems and Therapeutic Targeting
- Admission: Free
Add this event to my calendar
Click on "Create a calendar file" and your browser will download a .ics file for this event.
Microsoft Outlook: Download the file, double-click it to open it in Outlook, then click on "Save & Close" to save it to your calendar. If that doesn't work go into Outlook, click on the File tab, then on Open & Export, then Open Calendar. Select your .ics file then click on "Save & Close".
Google Calendar: download the file, then go into your calendar. On the left where it says "Other calendars" click on the arrow icon and then click on Import calendar. Click on Browse and select the .ics file, then click on Import.
Apple Calendar: The file may open automatically with an option to save it to your calendar. If not, download the file, then you can either drag it to Calendar or import the file by going to File >Import > Import and choosing the .ics file.
Eukaryotic cells maintain a balance of anabolic and catabolic pathways that are tuned to meet the demands of changing environments. The eIF2alpha kinase GCN2 is activated by amino acid starvation, while the protein kinase mTORC1 is activated when amino acids are replete. These nutrition-sensing pathways are linked through their influence on autophagy and lysosomal sorting. The VPS34-containing complexes are key components of these sorting pathways. Our work on GCN2, mTORC1 and VPS34 complexes is aimed at understanding the structural mechanisms that regulate these processes in control of growth, pathogenesis and cancer. The mTORC1 and VPS34 complexes are regulated by small G proteins that assemble on membrane surfaces.
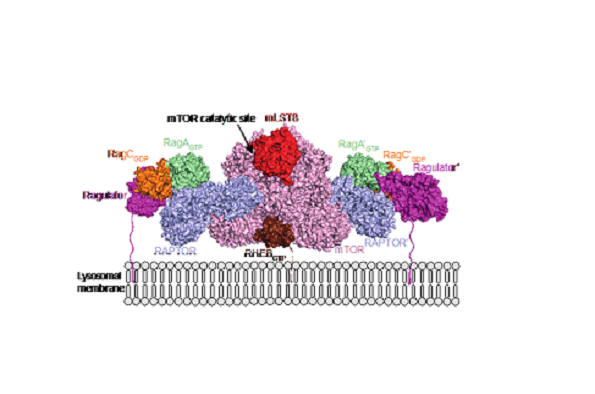
Heterodimeric Rag GTPases are key to activating the mTORC1 pathway in response to amino acids. We used X-ray crystallography to determine the structures of active forms of human Rag heterodimers and hydrogen/deuterium exchange mass spectrometry (HDX-MS) to map the mTORC1/Rag heterodimer interface and dynamics in solution. Our cryo-EM structure of a complex of mTORC1 with an active Rag heterodimer explains how oncogenic mutants activate the Rag heterodimer, how nucleotide binding is communicated between partners in the heterodimer, and why only one of the four states of the heterodimer activates mTORC1. Cryo-EM structures suggest that the primary role of Rag GTPase binding is to recruit mTORC1 to lysosomal membranes, in contrast to RHEB that activates the complex by an allosteric mechanism.
While mTORC1 is activated by amino acid abundance and promotes protein translation, nutrient stress activates GCN2, which initiates the Integrated Stress Response (ISR) and inhibits general translation. We have reconstituted this process in vitro, using purified components. This system shows that human GCN2 is potently stimulated by ribosomes. Using HDX-MS we showed that GCN2 recognises domain II of the uL10 subunit of the ribosomal P-stalk. The conserved 14 residue C-terminal tails (CTTs) of the P1 and P2 P-stalk proteins are also essential for GCN2 activation, and both the HisRS-like and kinase domains of GCN2 change conformation on binding the P-stalk complex. We propose that the P-stalk could link GCN2 activation to translational stress, leading to initiation of ISR.
