Matthew Smith from Bower Research Group, receives National 'Young Chemist Award’

Matthew Smith from the School's Department of Chemistry working in the Bower Research Group has been awarded the 'Young Chemist Award' from Scientific Update. For the award (one of just ten awarded nationally) Scientific Update received a large number of applications and the standard was said to be extremely high.
This award is aimed at those who are considering a career in process chemistry in industry (including pharmaceuticals, fine chemicals, agrochemicals, flavour & fragrance and speciality chemical industries) and will give researchers an opportunity to meet and network with key industrial leaders and discover potential career options.
As a result of winning this award Matthew has been granted:
- Free attendance at the conference
- 5 minutes to present his work – there will be an additional prize for the best presentation
- Poster board – there will be additional prizes for the best posters (open to all students) (Size A0 – portrait)
John Bower of the Bower Research Group had this to say about Matthew's award and research.
“Matt has developed really useful and interesting chemistry for the synthesis of pharmaceutically relevant nitrogen containing ring systems. His research has already resulted in two publications in Angewandte Chemie, which is a high impact general chemistry journal. This award recognises Matt’s achievements and is very well deserved.”
Matthew provided some information about himself and his research.
I am from Manchester and graduated from Imperial College London in 2020 where I obtained a First Class Honours MSci degree. During this time, I undertook a UROP placement with Dr Kafizas synthesising metal oxide films by chemical vapour deposition. For my final year project, I was working in Professor Christopher Braddock’s laboratory where I worked towards the formal total synthesis of anti-HIV compound (±)-peyssonol A.
Research
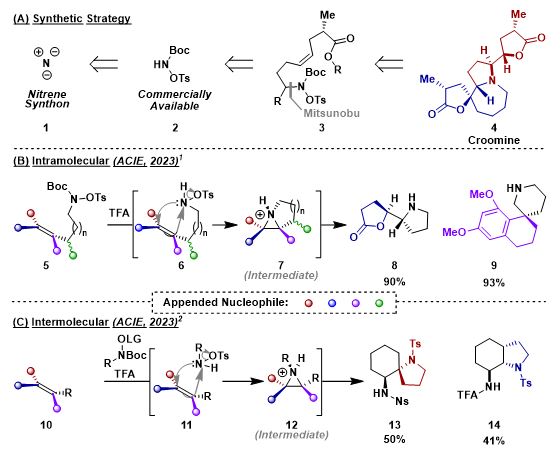
Robust synthetic methods towards functionalised, saturated heterocycles often require expensive metal catalysts and/or lack stereocontrol. My research focusses on accessing these desired compounds via a metal-free, stereospecific process without the need for hazardous oxidants. To achieve this, we sought after a gap in the current state of the art, utilising an aza-variant of the well-known m-CPBA epoxidation to form aziridines in-situ. Intramolecular (Scheme 1B)1 and intermolecular (Scheme 1C)2 alkene aziridination protocols which, followed by cascade intramolecular trapping, yielded complex heterocycles, stereospecifically, in a single step from linear precursors. We are now looking to apply the methodology in the target synthesis of magniflorine.
Scheme 1. (a) Synthetic strategy of 1,2-aminofunctionalisation towards complex heterocycles. (b) Intramolecular aziridination cascade approach to 1,2-aminofunctionalisation. (c) Intermolecular aziridination cascade approach to 1,2-aminofunctionalisation. Nu = Nucleophile. LG = Leaving Group.
References
- Zhu, Y.; Smith, M. J. S.; Tu, W.; Bower, J. F. Angew. Chem. Int. Ed. 2023, 62, e202301262.
- Smith, M. J. S.; Tu, W.; Bower, J. F. Angew. Chem. Int. Ed. 2023, 62, e202312797.