With your support The Pandemic Institute will:
Develop a state-of-the-art 12-bed overnight human challenge facility to test early medicines in human volunteers
The facility will enable our scientists to uncover critical information about the effectiveness of treatments, diagnostics and prevention approaches for a range of respiratory infections including COVID-19 and influenza. This infrastructure will benefit research in government and the wider biomedical sector, meaning global discoveries can be translated safely and more quickly for the people who need them most.
Expand the AGILE clinical trial platform
So that we can test many treatments in parallel and speed up testing by pooling data across studies. This approach– a world-first for infectious diseases – will be made fully open for scientists from across the globe. AGILE will test affordable and scalable antivirals for some of the biggest emerging threats.
Invest in an innovative “body on a chip” technology
This allows scientists to quickly replicate in miniature the complex environment of tissues such as the lung or liver. Through this, we can more confidently decide which treatments are most safe and effective, saving millions of pounds of future investment and ensuring only the medicines with the most life-saving potential are taken forward into studies in people.
We will accelerate our vaccine development programme
We will do this using novel approaches to study the body’s immune defences to priority pathogens. Liverpool’s vaccine development pipeline currently extends from preclinical studies all the way through to clinical trials, with “first in human” studies planned for new vaccines against Zika virus and Streptococcus pneumonia. Currently there are 15 pathogens in the programme; we will expand this to include more of the “priority pathogens” defined by the WHO, and also extend into new vaccine delivery mechanisms. This will include a wide range of industry collaborations.
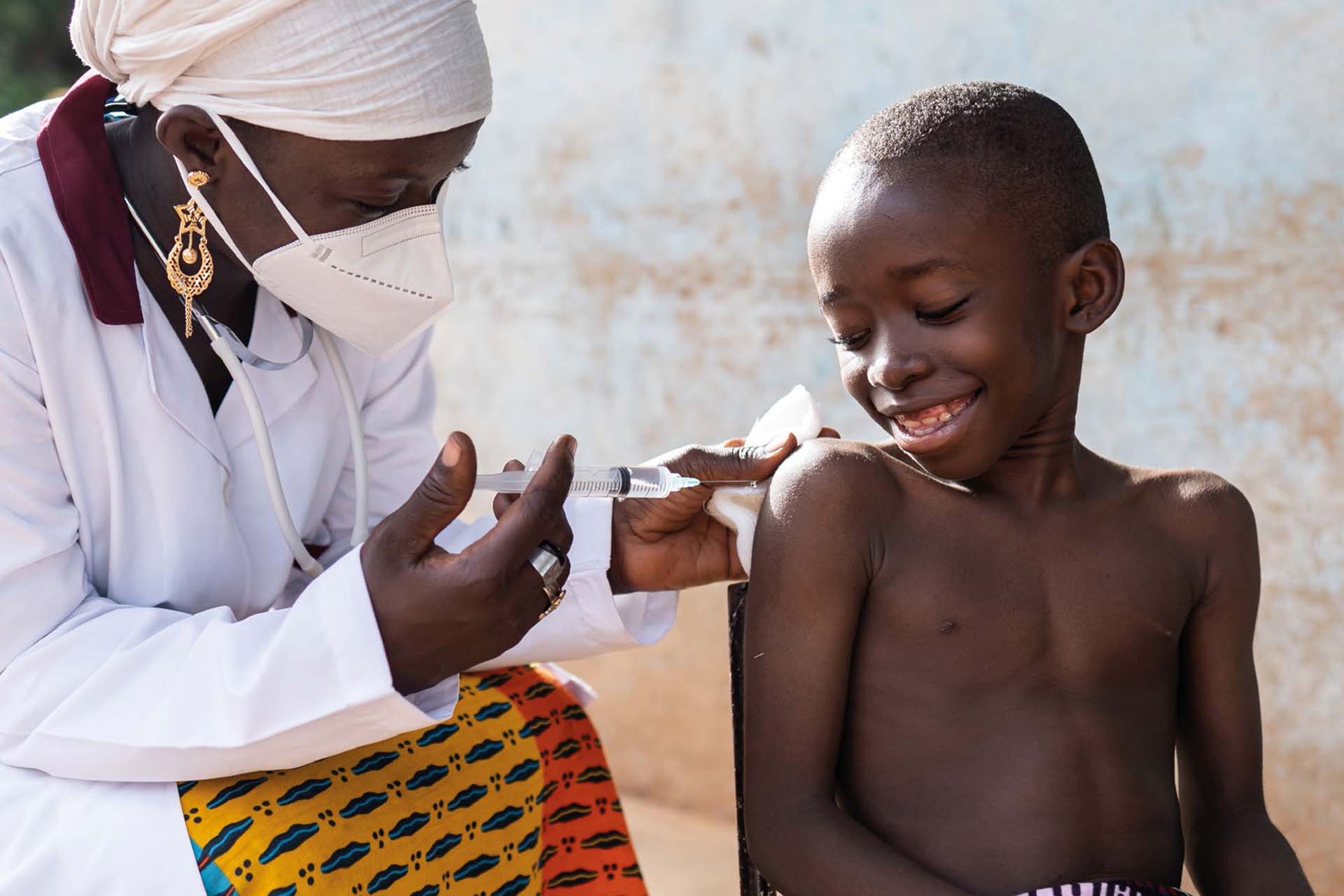
Develop International Vaccine Manufacturing Hubs
The lack of ‘COVID-19’ vaccines in low- and lower middle-income countries was one of the striking failures of the pandemic response. To provide fair access to vaccines against future emerging infections, we will enhance the research, development and manufacturing capabilities in these countries. Our first Vaccine Manufacturing Research Hub will be created in partnership with Kamuzu University of Health and Life Sciences, Blantyre, Malawi. It will reformulate and redevelop current vaccines to make them more readily available and useable in challenging overseas settings; create novel vaccine manufacturing technologies appropriate for use in resource poor-settings; and design economic modelling and decisional tools to assist vaccine development, distribution and use in low- and lower middle-income countries.
Back to: The Pandemic Institute