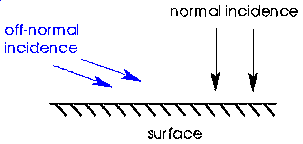
We're really asking what effect does each of the two components of momentum have, i.e. the momentum perpendicular to the surface, and the momentum parallel to the surface. In order to answer this, we need to introduce some terminology: It is customary in gas-surface dynamics to discuss angular dependence in terms of "energies" and energy scaling. So instead of normal momentum, we would say normal energy. In fact the normal energy is just the energy associated with the normal component of momentum.
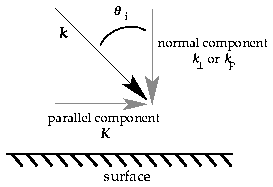 |
Using Ep (note should be epsilon but the browser can't
support this) for the normal energy, it is easy to see from the figure that
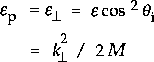 where M is the mass of the molecule. |
By energy scaling we mean what is the value of n such that
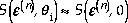 where
where
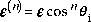
What these equations mean is that if you take the dissociation probability at off-normal incidence and plot it not as a function of E, but as a function of E(n) instead, then it will lie exactly on top of the normal incidence data, for which E(n) = E anyway since the angle of incidence is zero.
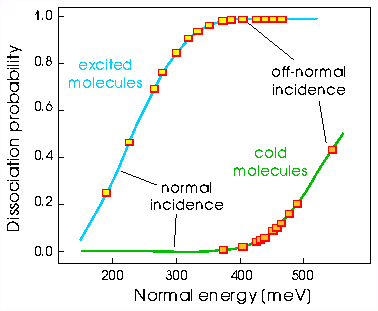
Normal Energy Scaling
As an example, let's take n = 2 known as normal energy scaling, perhaps the most common
example.
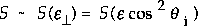
So, in other words, graphing dissociation/sticking probability versus normal energy will cause all the points at whatever angles of incidence to collapse on to one line, as shown in the example below.