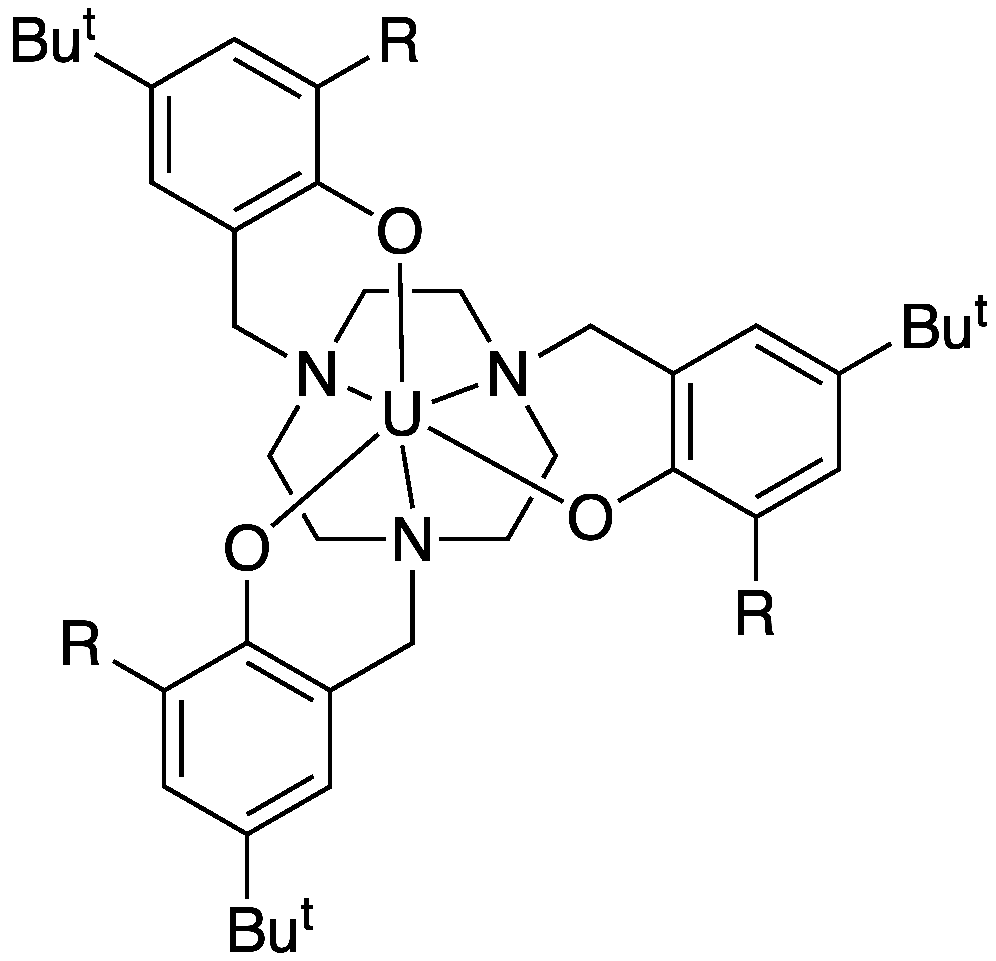
 |
The hexadentate ligand causes steric crowding at the U(III) centre, particularly when R is adamantyl. This highly crowded U(III) complex shows some unique chemistry |
| CO2 is reduced by the U(III) atom to form a radical anion coordinated to U(IV). When R=adamantyl, this complex is so sterically hindered that no further reaction of CO2 can occur | When R=But the CO2 complex that is formed initially is reduced further by excess U(III) complex in solution. CO is eliminated and an oxo-bridged dimer is formed. This reaction is prevented where R=adamantyl due to steric hindrance |