Click on the links to display the structures
|
|
 |
 |
|
[La(acac)3(H2O)2] exists as a dihydrate in order to fill coordination sphere of the large La3+ion.
The much smaller Yb3+ ion forms a monomeric anhydrous complex [Yb(acac)3] |
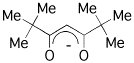
[Pr(thd)3]2 exists as a dimer whereas [Lu(thd)3] is a monomer due to the smaller size of the Lu3+ ion.
|
Coordination number can be increased by forming an anionic complex with
four diketonate ligands. [Eu(dbm)4]- is a good example of an
8-coordinate complex [Eu(dbm)4]- |