|
|
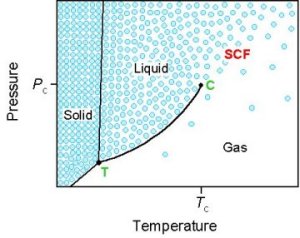
Supercritical Fluids |
|
|
1. Introduction Most of our research involves polymer chemistry, in particular the use of supercritical fluids (SCFs) as novel solvents for polymer synthesis and processing. As society places increasing emphasis on environmental issues, chemists face the challenge of developing more responsible chemical methods, as embodied by the popular phrase ‘green technology.’ Supercritical carbon dioxide (scCO2) is an attractive alternative solvent because it is inexpensive, non-toxic, and non-flammable (A.I. Cooper, J. Mater. Chem., 2000 10, 207). Moreover, separation of solute from solvent is simplified because CO2 reverts to a gas upon depressurisation. Some examples of areas where scCO2 has found applications are: ˇ Extraction ˇ Separation ˇ Polymer processing ˇ Organic synthesis ˇ Particle formation ˇ Microlithography |
|
|
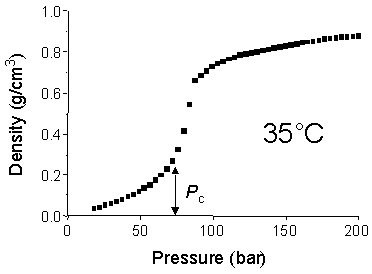
2. Variable Density Unlike conventional liquid solvents, SCFs are highly compressible and the density can be varied over a wide range by changing pressure. The graph on the right shows how the density of pure CO2 changes as the pressure is increased to 200 bar at a constant temperature of 35ēC. Note that there is a sudden increase in density as the critical pressure (Pc, 73.8 bar) is approached. By ‘tuning’ the density (and therefore the solvent properties) of supercritical fluid solvents, one can control a number of variable such as: ˇ Solubility and phase behaviour ˇ Reaction rate ˇ Reaction pathway (e.g., stereochemistry, product selectivity) ˇ Particle size |
|
|
3.
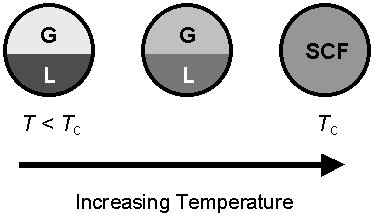
Compressed Gas or Expanded Liquid? A supercritical fluid can be considered as a highly compressed gas (see previous graph) or as an expanded liquid (see diagram on right). Both viewpoints are useful for understanding the physical properties of SCF solvents. A supercritical fluid can exhibit liquid-like densities (>0.7 g/cm3) while having viscosity that is intermediate between a liquid and a gas. This unique combination of high density and low viscosity makes SCF solvents particularly attractive for processes such as: ˇ Impregnation and dyeing ˇ Organic reactions (particularly those which are mass transfer limited) ˇ Coatings ˇ Microlithography |
|
Books on Supercritical Fluids(1) P.G. Jessop, W. Leitner, Chemical Synthesis using Supercritical Fluids; Wiley, VCH: Weinheim, 1999 (2) M.A. McHugh, V.J. Krukonis, Supercritical Fluid Extraction; Butterworth-Heinemann: Stoneham, MA, 1994 |
|