|
|
Porous Polymers |
|
Macroporous Polymer Monoliths
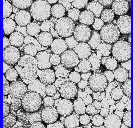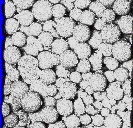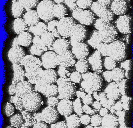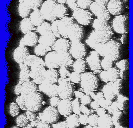
The synthesis of porous polymers is important in a wide range of applications. Our research in this area involves the use of scCO2 as a solvent for the synthesis of macroporous cross-linked polymers (see micrograph on right). Our results suggest that CO2 may be a very useful solvent for producing such materials, and it has been demonstrated that one can synthesise macroporous polymer monoliths with a wide range of pore sizes (A.I. Cooper and A.B. Holmes, Adv. Mater.,1999 11, 2170). (Click here for a schematic of this process.) It is possible to generate polymers with very narrow pore size distributions using this method. We are currently exploring ways in which we can exploit the unique physical properties of supercritical fluid solvents in order to generate novel materials. A particular advantage is the ease with which the supercritical porogen can be removed from the polymer matrix. |
|
|
Reviews on Porous Polymer Monoliths: (1) E.C. Peters, F. Svec and J.M.J. Frechet, Adv. Mater. 1999 11, 1169 (2) F. Svec
and J.M.J.
Frechet, Science 1996 273, 205 More Porous Materials
Synthesised using CO2: (1) C. Shi, Z. Huang, S. Kilic, J. Xu, R. M. Enick, E. J. Beckman, A. J. Carr, R. E. Melendez and A. D. Hamilton, Science 1999 286, 1540 (2) S. M. Howdle, M. S. Watson, M. J. Whitaker, V. K. Popov, M. C. Davies, F. S. Mandel, J. D. Wang and K. M. Shakesheff, Chem. Commun. 2001, 109 |
|
Macroporous
Beads
We have also extended this technique to the preparation of macroporous polymer beads by suspension polymerisation using scCO2 as the porogen (C.D. Wood and A.I. Cooper, Macromolecules, 2001 34, 5). An example of these beads is shown in the animated 3D microscope image on right (obtained with help from Dr Ian Hopkinson, University of Cambridge). This work was initiated by Mr Colin Wood, a PhD student cosponsored by Avecia Ltd. Our aim here is to tune the pore size in the materials by changing the density of the SCF phase, thus employing the unique physical characteristics of SCFs in order to gain control over material properties. A poster on this work can be downloaded here: CDW_Poster.jpg (0.84 MB) |
|
|
Reviews on Suspension Polymerisation: (1) H.G. Yuan, G. Kalfas and W.H. Ray, J. Macromol. Sci., Rev. Macromol. Chem. Phys. 1991 C31, 215 (2) (2) E. Vivaldo-Lima, P.E. Wood, A.E. Hamielec, A. Penlidis, Ind. Eng. Chem. Res. 1997 36, 939 |
|
Emulsion
Templating
Very recently, we have developed a new method for the preparation of highly porous materials by emulsion templating using supercritical fluids. An electron micrograph of one of these materials is shown on the right (click to expand). The materials are removed from the reaction vessel as continuous monoliths which conform to the shape of the reaction vessel, as can be seen from the picture below right. (The coin, a British 2p piece, is shown for scale.) This work was initiated by Ms Rachel Butler (PhD student, cosponsored by Bradford Particle Design Plc) and Ms Cait Davies (then an MChem student, now studying for a PhD in our group). Since the template phase is CO2, no organic solvent residues are left in the materials at the end of the reaction. Furthermore, the process is not limited to materials that can be foamed (i.e., rigid inorganic frameworks are also possible). For further details, see: R.
Butler, C.M. Davies and A.I. Cooper, Adv. Mater., 2001 13, 1459 Other Recent Papers on Emulsion Templating: (1) A. Barbetta, N.R. Cameron and S.J. Cooper, Chem. Commun. 2000, 221 (2) V.N. Manoharan, A. Imhof, J.D.
Thorne and D.J. Pine, Adv. Mater. 2001 13, 447 |
|
|
|
|