|
|
Dendrimers |
|
|
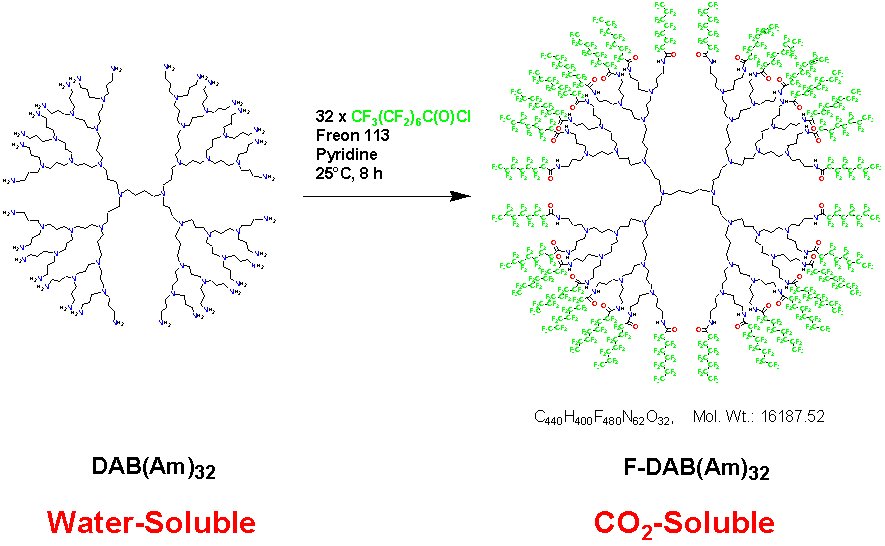
A large number of polar species (e.g., ionic compounds, biomolecules) exhibit very low solubility in scCO2, thus limiting the use of this solvent. To address this problem, CO2-soluble dendrimers were developed for the extraction of polar compounds (e.g., ionic dyes) from water into CO2 (A.I. Cooper et al., Nature, 1997 389, 368; see reaction scheme below). The animated image on the right shows this process in action. In the presence of the CO2-soluble dendrimer, a water-soluble dye (methyl orange) is extracted into the CO2 phase. In the absence of the dendrimer, the dye is completely insoluble in CO2. Systems of this type have great potential in applications such as extraction, phase transfer, and catalysis (see for example: E.LV. Goetheer et al., Ind. Eng. Chem. Res. 2000 39, 4634). Recent Dendrimer Reviews: (1) A.W. Bosman, H.M. Jansen and E.W. Meijer, Chem. Rev., 1999 99, 1665 (2) M.W.P.L. Baars and E.W. Meijer, Top. Curr. Chem., 2000 210, 131 (3) M. Fischer and F. Vogtle, Angew. Chem. Int. Ed., 1999 38, 885 |
Upper Layer = CO2 |