
Dr Nordine Helassa BSc(Hons), MRes, PhD, FHEA
Senior Lecturer - BHF Research Fellow Biochemistry, Cell and Systems Biology
- +44 (0)151 794 4191
- Work email nhelassa@liverpool.ac.uk
- Personal Websitehttps://nhelassa.wixsite.com/helassa-lab
- About
- Research
- Publications
- Teaching
- Professional Activities
Research
Project 1: Calmodulin and ion channels in cardiovascular diseases
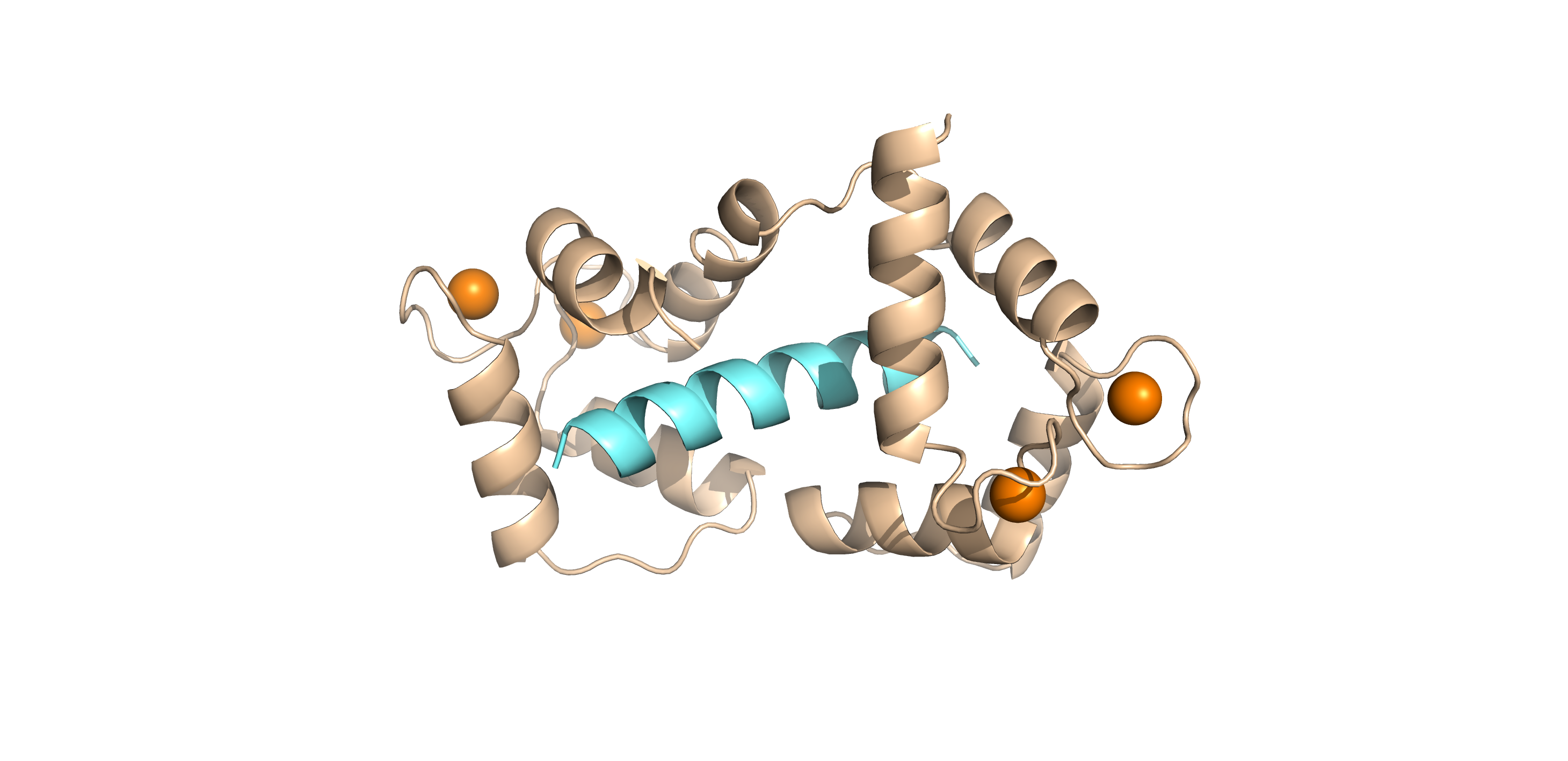
Long QT syndrome (LQTS) and catecholaminergic polymorphic ventricular tachycardia (CPVT) are inherited conditions that disturb heart rhythm and can cause life-threatening arrhythmias (e.g. sudden cardiac death). Recent studies have identified mutations in the major calcium sensor calmodulin (CaM) that are associated with cardiac arrhythmia susceptibility suggesting that CaM dysfunction is a key driver of the molecular aetiology of these diseases. However, the detailed molecular mechanism leading to irregular heartbeats in LQTS and CPVT remains unclear. We investigate the role of CaM and the effect of the disease-causing mutations in the regulation of calcium fluxes during cardiac excitation-contraction coupling using a multidisciplinary approach (protein biophysics, structural biology, cellular imaging and electrophysiology). Through a comprehensive structure-function analysis characterising the effect of CaM mutations on its interaction network relevant to cardiac muscle contraction, we aim to develop a detailed mechanistic understanding of congenital heart dysfunctions which will open ways to future therapies.
Project 2: Development of fluorescent probes for biological applications
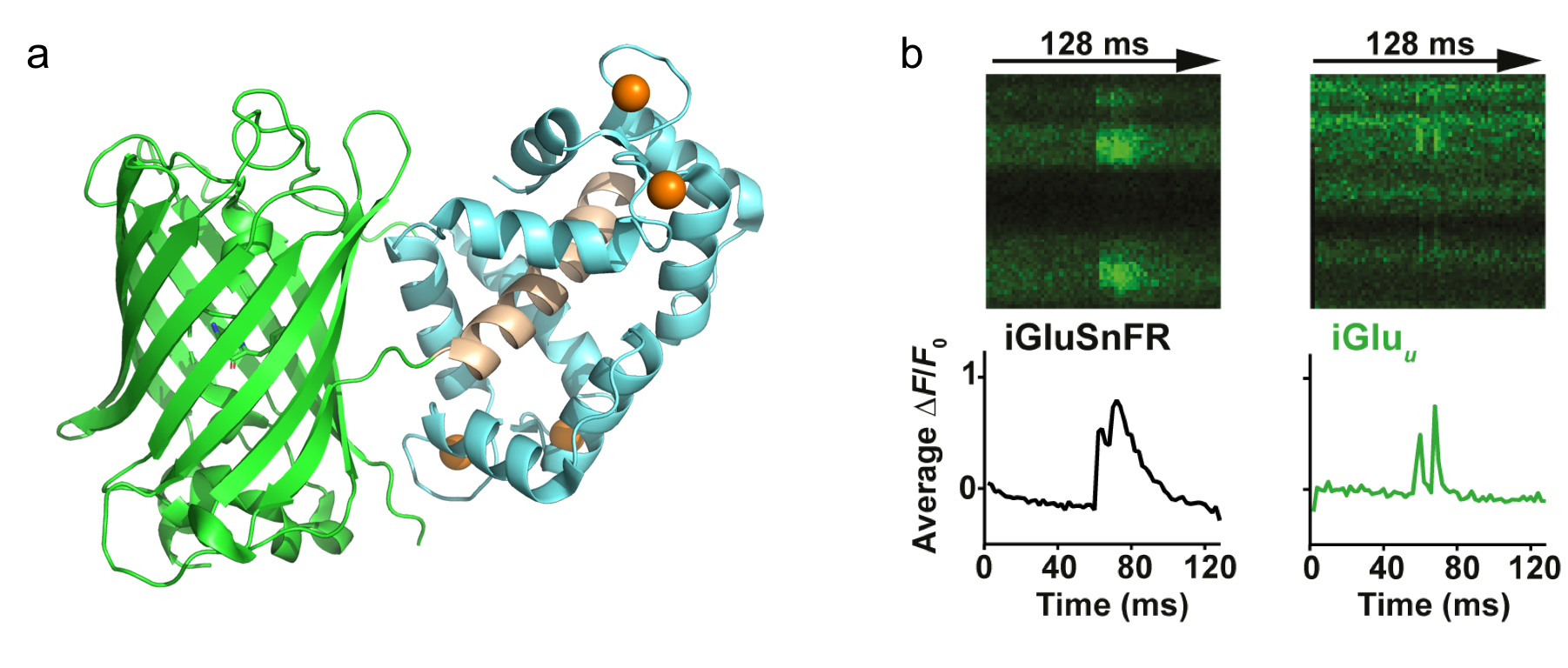
- Calcium and glutamate indicators
Green Fluorescent-Calmodulin Proteins (GCaMPs) and genetically-encoded fluorescent glutamate indicator iGlu-‘sniffer’ (iGluSnFR) have been the reporters of choice for visualising neuronal network activity and neurotransmitter glutamate release in vivo. However, their slow rise and decay rates limit the temporal resolution of high frequency calcium transients and glutamate release. To improve the kinetics of GCaMPs and iGluSnFR, we mutated selected amino acid residues coordinating calcium and glutamate at the binding sites. We aim to characterise the improved indicators in terms of fluorescence dynamic range, affinity, association and dissociation kinetics and pH/temperature dependence, using a range of biophysical techniques. The newly developed probes will be more suitable for monitoring fast, high frequency calcium transients e.g. action potentials (GCaMPs) and glutamate neurotransmitter release (iGluSnFR) in vivo.
-Glucose indicators
Both lung disease and elevation of blood glucose are associated with increased glucose concentration (from 0.4 to ~4.0 mM) in the airway surface liquid (ASL). This perturbation of ASL glucose makes the airway more susceptible to infection by respiratory pathogens. ASL is minute (~1 μl/cm2) and the measurement of glucose concentration in the small volume ASL is extremely difficult. Therefore, we sought to develop a fluorescent biosensor with sufficient sensitivity to determine glucose concentrations in ASL in situ using environmentally sensitive fluorophores coupled by chemical labeling to a glucose/galactose-binding protein (GBP). The fluorescent GBP generated will be a useful tool to monitor glucose homoeostasis in the lung.
Project 3: Hippocalcin in neurodegenerative disease
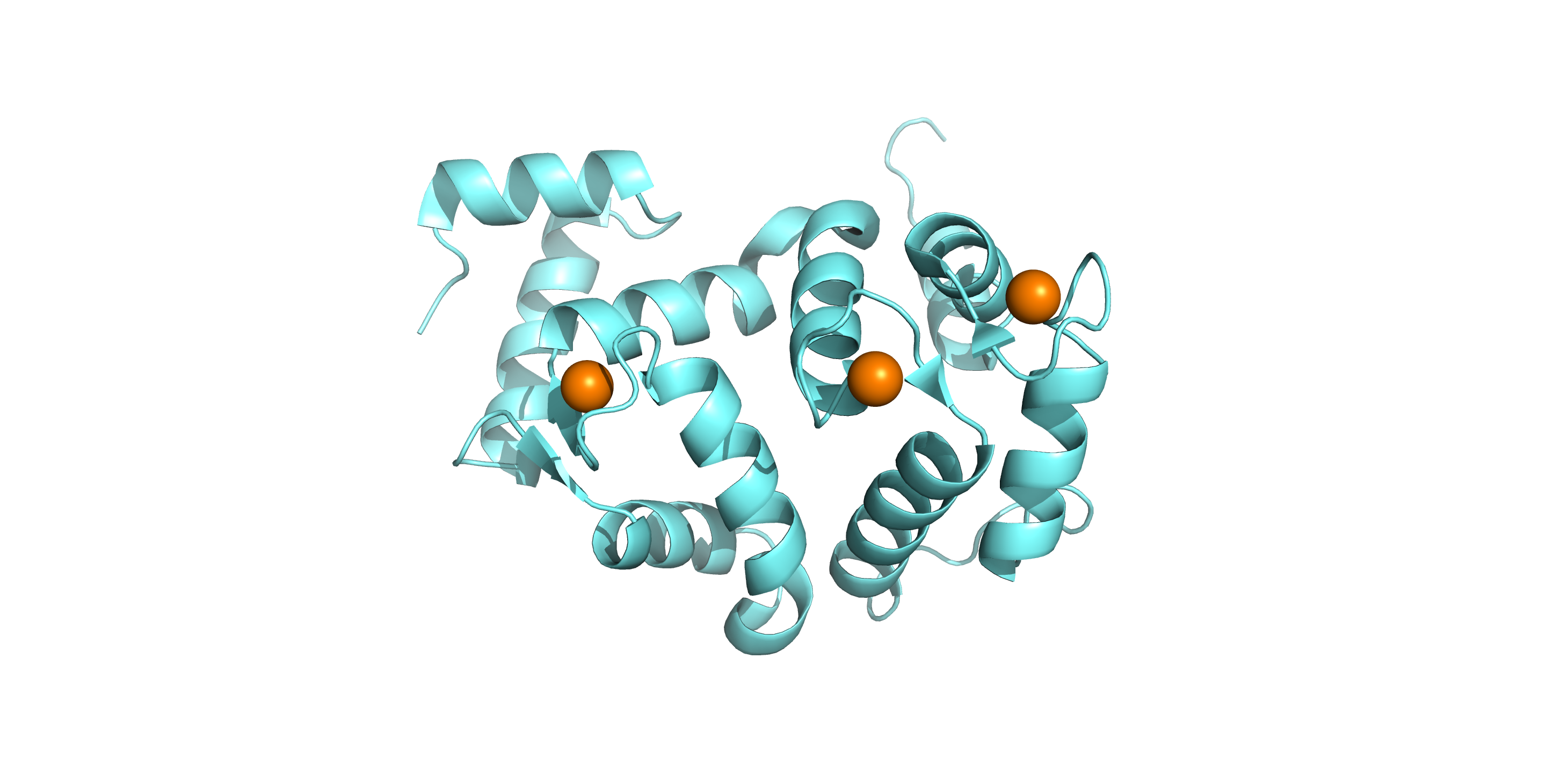
Dystonia is a neurological movement disorder that causes muscle spasms and contractions. It is characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive movements and painful postures than can be accompanied by dystonic tremor. With the advent of next-generation sequencing technologies, mutations in the neuronal calcium sensor hippocalcin have been identified as the genetic cause of primary isolated dystonia (DYT2 dystonia). However, the effect of these mutations on the physiological role of hippocalcin has not yet been elucidated. Using a combination of biophysical, structural biology and cell biology techniques, we aim to determine the effect of these mutations on hippocalcin cellular functions and calcium signalling in DYT2 dystonia.
Research Grants
Characterisation of novel cardiac troponin I mutants associated with human hypertrophic cardiomyopathy
BRITISH HEART FOUNDATION (UK)
December 2021 - November 2024
Wellcome Trust Four-Year PhD Studentship Programme
WELLCOME TRUST (UK)
October 2017 - March 2022
Investigating the role of calmodulin in cardiac arrhythmia (CALM HEART)
BRITISH HEART FOUNDATION (UK)
February 2018 - January 2025
Research Collaborations
Prof Martin Morad
External: Medical University of South Carolina
Cardiac electrophysiology
Dr Lowri Thomas
External: Cardiff University
Ryanodine receptors physiology