Nanoscience
The overall scope of the research programme is the exploration of the fundamental physics underlying and underpinning developments in a range of applied areas including nanotechnology, sensor development, clean energy sources and environmentally friendly materials.
| Dr Steve Barrett | Department of Physics |
| Dr Yvonne Grunder | Department of Physics, Stephenson Institute for Renewable Energy |
| Dr Frank Jaeckel | Department of Physics, Stephenson Institute for Renewable Energy |
| Professor Chris Lucas |
Department of Physics, Stephenson Institute for Renewable Energy |
| Professor Ronan McGrath |
Department of Physics, Surface Science Research Centre |
| Dr Hem Raj Sharma |
Department of Physics, Surface Science Research Centre |

In-situ X-Ray Scattering Studies of Electrochemical Systems
In the last decade, synchrotron surface x-ray diffraction has been a critical tool for determining the potential dependence and stability of specific surface structures in electrolyte under reaction conditions. In-situ diffraction is a unique tool that allows structural characterisation of the interfacial atomic structure including subsurface and ordering on the electrolyte side of the interface.
Read more...

Electrochemical characterisation of solid-liquid interfaces
Electrochemical investigation is carried out in our new electrochemical laboratories in the Stephenson Institute for Renewable Energy. We currently have a couple of measuring stations allowing the study of electrochemical surface processes by electrochemical methods (e.g. cyclic voltammetry, chronoamperometry).
Read more...

Epitaxy on Quasicrystal Surfaces and Intermetallics in Catalysis
Quasicrystals are new type of solids that differ from other two known forms, crystal and amorphous, by possessing long range order without periodicity. They are of specific stoichiometry and often exhibit crystallographically forbidden rotational symmetries such as tenfold and fivefold.
Read more...

Hybrid Nanomaterials
Nanomaterials exhibit properties significantly different from their bulk counterparts due to quantum confinement effects. This allows tuning their electronic and optical properties beyond what is possible in the bulk.
Read more...
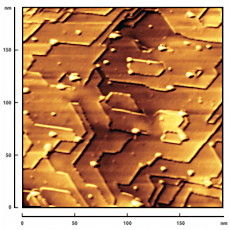
Scanning Microscopy and Electron Diffraction
Scanning microscopy techniques are used extensively in nanoscience. Scanning Probe Microscopy (SPM) is a generic term encompassing Scanning Tunnelling Microscopy (STM), Atomic Force Microscopy (AFM), Scanning Near-Field Optical Microscopy (SNOM) and related techniques. In addition, Scanning Auger Microscopy (SAM), Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) are often used in nanoscale characterisation of materials. Images generated from all of these microscopy techniques need processing and analysis to extract quantitative information.
Read more...
Back to: Department of Physics
